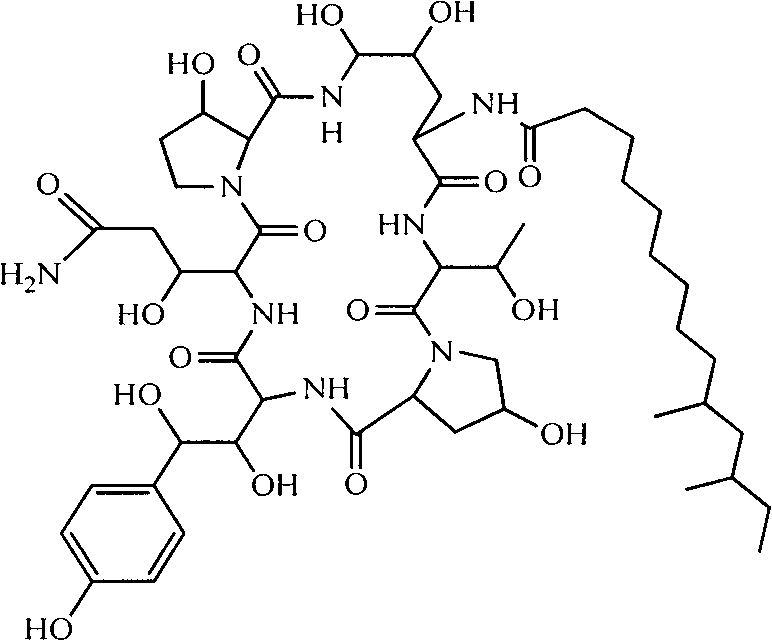
Preparation method of high-purity pneumocandin B0
A lung capsule Kangding, high-purity technology, applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry and other directions, can solve the problems of unsuitability for industrial production, cumbersome processes, and high costs, and achieve shortened process cycles. The effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
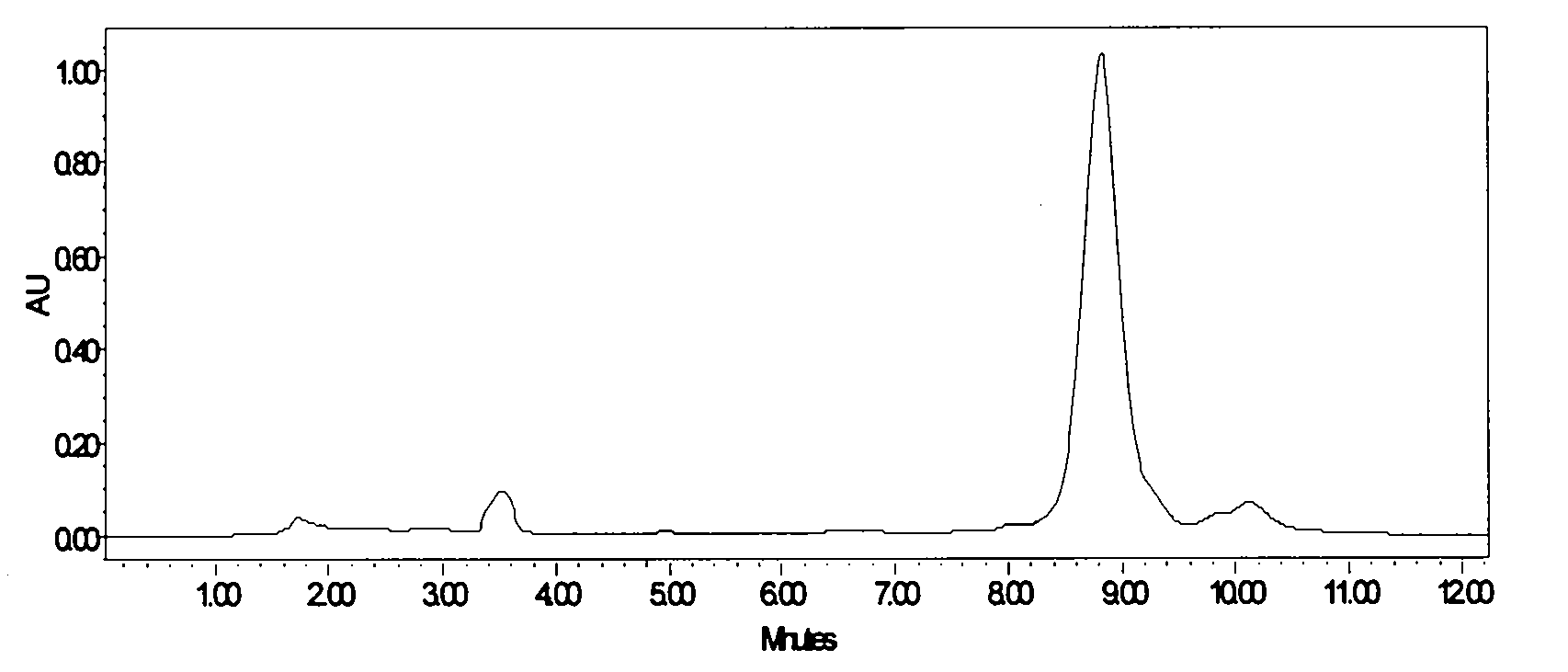
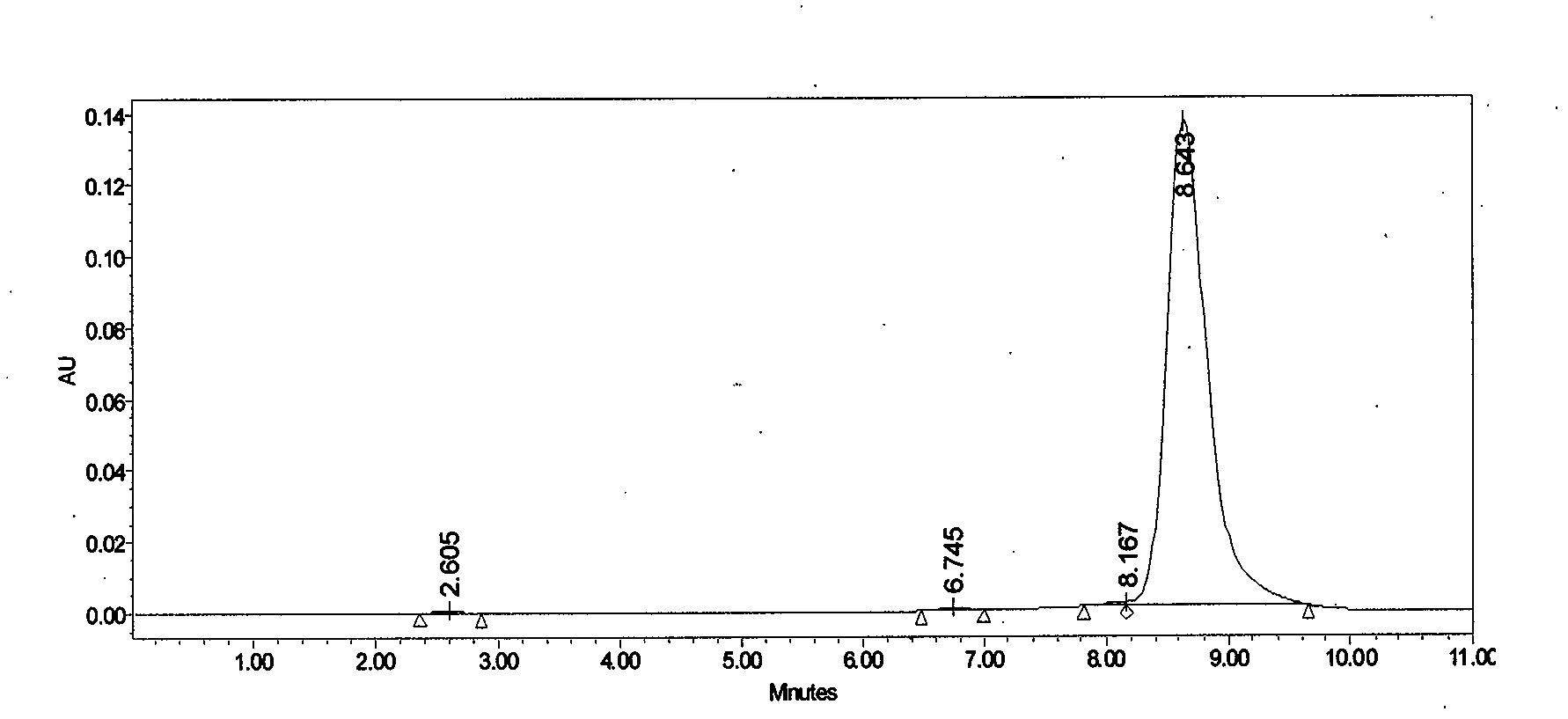
[0039] Take Lung Sac Kangding B 0 Fermentation broth 10.0L, fermentation broth unit 832μg / mL. 100 g of perlite was added to the fermentation broth, stirred for 30 minutes and then vacuum filtered to obtain 2.0 kg of fermentation culture. Add 8L of industrial ethanol to the fermentation culture, stir mechanically for 1 hour, and then vacuum filter to collect the extract. The extract was decolorized through a D303 resin column with a diameter-to-height ratio of 1:6, the resin loading capacity was 500 mL, and the flow rate was 1000 mL / h. Collect the decolorized solution, adjust the concentration of the solvent to 30%, and introduce the HZ830 resin column with a diameter-to-height ratio of 1:6 for adsorption. The resin loading capacity is 400 mL, and the flow rate is 800 mL / h. The saturated resin was purified and washed with 40% ethanol, and then desorbed with 80% ethanol at a flow rate of 200mL / h. The desorption solution was concentrated, extracted with ethyl acetate, and acet...
Embodiment 2
[0041] Take Lung Sac Kangding B 0 Fermentation broth 10.0L, fermentation broth unit 762μg / mL. Add 300g of perlite to the fermentation broth, stir for 1 hour and then vacuum filter to obtain 2.2kg of fermentation culture. Add 13.2L of industrial ethanol to the fermentation culture, stir mechanically for 2 hours, and then vacuum filter to collect the extract. The extract was decolorized through a D303 resin column with a diameter-to-height ratio of 1:6, the resin loading capacity was 500 mL, and the flow rate was 1000 mL / h. Collect the decolorized solution, adjust the concentration of the solvent to 40%, and introduce the HZ830 resin column with a diameter-to-height ratio of 1:6 for adsorption. The resin loading capacity is 400 mL, and the flow rate is 800 mL / h. The saturated resin was purified and washed with 50% ethanol, and then desorbed with 90% ethanol at a flow rate of 200 mL / h. The desorption solution was concentrated, extracted with ethyl acetate, and acetone was crys...
Embodiment 3
[0043] Take Lung Sac Kangding B 0 Fermentation broth 20.0L, fermentation broth unit 973μg / mL. Add 500 g of perlite to the fermentation broth, stir for 1 hour and then vacuum filter to obtain 4.5 kg of fermentation culture. Add 19L of industrial ethanol to the fermentation culture, mechanically stir for 1 hour, and then vacuum filter to collect the extract. The extract was decolorized through a D303 resin column with a diameter-to-height ratio of 1:6, the resin loading capacity was 1000 mL, and the flow rate was 2000 mL / h. Collect the decolorized solution, adjust the concentration of the solvent to 30%, and introduce the HZ830 resin column with a diameter-to-height ratio of 1:6 for adsorption. The resin loading is 1000 mL, and the flow rate is 2000 mL / h. The saturated resin was purified and washed with 40% ethanol, and then desorbed with 90% ethanol, and the desorption flow rate was 500mL / h. The desorption solution was concentrated, extracted with ethyl acetate, and crystall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com