Hybridoma cell strain generating anti-human NGAL specific monoclonal antibody, monoclonal antibody generated by same and application
A hybridoma cell line, monoclonal antibody technology, applied in the direction of anti-animal/human immunoglobulin, microorganism-based methods, microorganisms, etc., can solve the problems of difficult clinical application and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of NGAL recombinant protein
[0039] 1. Entire gene acquisition of NGAL coding sequence
[0040] According to the NGAL gene sequence (NM_005564.3) provided by GeneBank, the pseudo-cloning sequence of the human neutrophil gelatinase-related lipocalin gene was determined, as shown in SEQ ID NO: 2:
[0041] Design NGAL full-length primers: shown in SEQ ID NOs: 3 and 4, respectively
[0042] SEQ ID NO: 3: NGAL-1: 5'-TAAGGTACCCATGCCCCTAGGTCTCCTG-3'
[0043] SEQ ID NO::4:NGAL-2:5'-GGTGGAATTTTAGCCGTCGATACACTG-3'
[0044] The primers respectively contain restriction sites: NGAL-1: Acc651; NGAL-2: EcoRI, synthesized by Invitrogen Company.
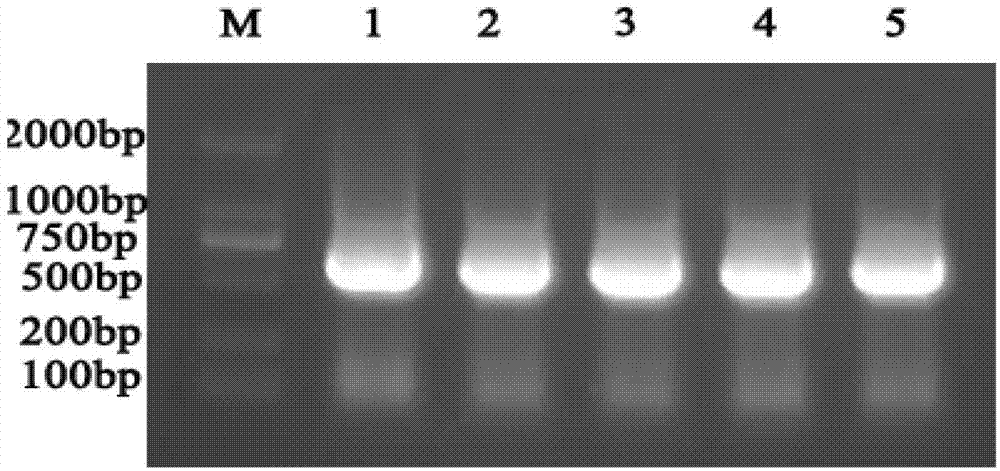
[0045] Total leukocyte RNA was extracted by Trizol Reagent method, leukocyte cDNA was obtained by reverse transcription, and human NGAL was obtained by PCR amplification. A PCR system was established, and PCR was performed at different annealing temperatures. Take 25 μL of PCR products for analysis by 1% TAE electr...
Embodiment 2
[0059] Example 2 Bioinformatics analysis and identification of immunogens
[0060] 1. Find the amino acid sequence of the NGAL protein from GENEBANK, which consists of 198 amino acid residues.
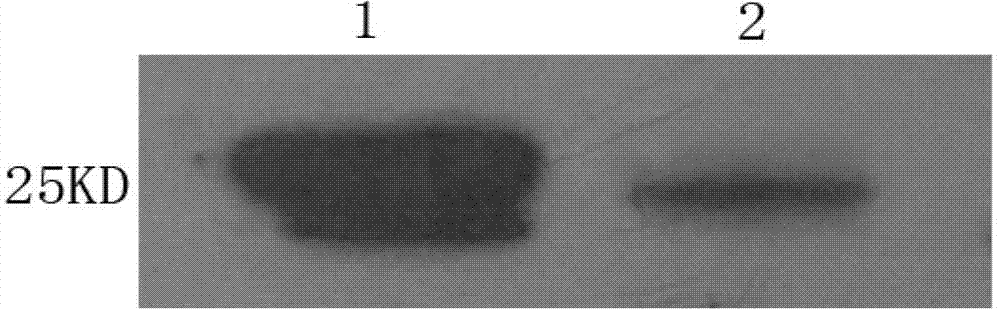
[0061] 2. Online bioinformatics glycosylation prediction analysis The 86th amino acid in NGAL protein may be glycosylated. In order to obtain the best specific monoclonal antibody that can recognize the natural protein, the full-length NGAL protein expressed in 293 cells was selected. As an immunogen, its amino acid sequence is the same as SEQ ID NO:1.
Embodiment 3
[0062] Example 3 Preparation of anti-NGAL monoclonal antibody
[0063] 1. Preparation of NGAL monoclonal antibody
[0064] The recombinant full-length NGAL protein was emulsified with an equal amount of Freund's complete adjuvant, and the antigen was emulsified by double-syringe mutual push method. After the emulsification was completed, 5 Balb / c mice of about 8 weeks of age were given basal immunization (100 μg / mouse) by subcutaneous multi-point injection and intraperitoneal injection. After 2 weeks, NGAL whole antigen was emulsified with the same amount of incomplete Freund's adjuvant, and 5 Balb / c mice were boosted by subcutaneous injection and intraperitoneal injection (100 μg / mouse); 2 Weeks later, the same method was used for an additional immunization, and 7 days later, the mice were sacrificed to remove the spleen, and the spleen cells were fused.
[0065] The fusion process is to mix spleen cells and myeloma cells (Sp2 / 0) at a ratio of 8:1, and use polyethylene gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com