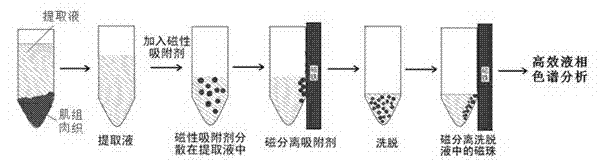
Method for enriching nature tetracycline antibiotics in aquatic products
A technology for tetracyclines and aquatic products, applied in chemical instruments and methods, material separation, instruments, etc., can solve the problems of increasing bacterial resistance and affecting the health of consumers, achieving fast enrichment speed and high recovery rate of sample extraction , the effect of less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
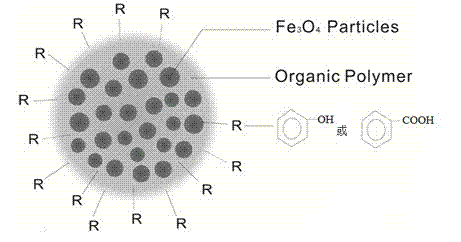
[0029] Example 1 Synthesis of polystyrene magnetic spheres modified with phenol or benzoic acid functional groups (particle size: 70 nm)
[0030] Referring to the literature (Yan F, Li J, Zhang J, Liu F, Yang W. J. Nanopart. Res., 2009, 11(2):289~296), the chemical co-precipitation method was used to synthesize superparamagnetic Fe 3 o 4 nanoparticles, and Fe 3 o 4 The surface oil of the nanoparticles was acidified, washed with absolute ethanol and then blown dry with nitrogen; take 5 g of the surface oil acidified Fe 3 o 4 Nanoparticles, suspended Fe in 5 mL of styrene-hexadecane mixture with a volume ratio of 15:1 3 o 4 Nanoparticles, ultrasonic 5 min to form a magnetic fluid, transfer the magnetic fluid to 400 mL, 0.01M NaHCO containing 0.5% SDS 3 In the solution, 300 W (25KHz) power continued ultrasonic emulsification for 30 minutes to form a miniemulsion; add 0.1g K to the above miniemulsion 2 S 2 o 8 After reacting at 70°C for 20 min, add 0.5 g of p-vinylphenol ...
Embodiment 2
[0031] Example 2 Synthesis of polystyrene magnetic spheres modified with phenol or benzoic acid functional groups (particle size: 100 nm)
[0032] Referring to the literature (Yan F, Li J, Zhang J, Liu F, Yang W. J. Nanopart. Res., 2009, 11(2):289~296), the chemical co-precipitation method was used to synthesize superparamagnetic Fe 3 o 4 nanoparticles, and Fe 3 o 4 The surface oil of the nanoparticles was acidified, washed with absolute ethanol and then blown dry with nitrogen; take 5 g of the surface oil acidified Fe 3 o 4 Nanoparticles, suspended Fe in 5 mL of styrene-hexadecane mixture with a volume ratio of 15:1 3 o 4 Nanoparticles, ultrasonic 5 min to form a magnetic fluid, transfer the magnetic fluid to 400 mL, 0.01M NaHCO containing 0.4% SDS 3 In the solution, 250 W (25KHz) power continued ultrasonic emulsification for 30 minutes to form a miniemulsion; add 0.1g K to the above miniemulsion 2 S 2 o 8 After reacting at 70°C for 10 min, add 0.5 g of p-vinylpheno...
Embodiment 3
[0033] Example 3 Synthesis of polystyrene magnetic spheres modified with phenol or benzoic acid functional groups (particle size: 200 nm)
[0034] Referring to the literature (Yan F, Li J, Zhang J, Liu F, Yang W. J. Nanopart. Res., 2009, 11(2):289~296), the chemical co-precipitation method was used to synthesize superparamagnetic Fe 3 o 4 nanoparticles, and Fe 3 o 4 The surface oil of the nanoparticles was acidified, washed with absolute ethanol and then blown dry with nitrogen; take 5 g of the surface oil acidified Fe 3 o 4 Nanoparticles, suspended Fe in 5 mL of styrene-hexadecane mixture with a volume ratio of 15:1 3 o 4 Nanoparticles, ultrasonic 5 min to form a magnetic fluid, transfer the magnetic fluid to 400 mL, 0.01M NaHCO containing 0.3% SDS 3 In the solution, 200 W (25KHz) power continued ultrasonic emulsification for 30 minutes to form a miniemulsion; add 0.1g K to the above miniemulsion 2 S 2 o 8 After reacting at 70°C for 10 min, add 0.5 g of p-vinylpheno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com