Biodegradable medical adhesive, and preparation method and purpose thereof
An adhesive, cyanoacrylic acid technology, applied in nitrile copolymer adhesives, ester copolymer adhesives, non-polymer organic compound adhesives, etc., can solve problems such as difficult degradation and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0096] Preparation Example 1: Synthesis of Bisα-cyanoacrylate PLA-PEG-PLA
[0097] The synthesis of bisα-cyanoacrylate compounds is basically similar, and the references (US3975422, 4012402, 4041061) are synthesized in a similar way. In this example, bisα-cyanoacrylate PLA-PEG-PLA is taken as an example for description.
[0098] The structure of bisα-cyanoacrylate PLA-PEG-PLA is as follows:
[0099]
[0100] Its unique intermediate segment PLA-PEG-PLA block copolymer (1-1) synthesis method is as follows:
[0101]
[0102] 10.13g PEG1000 (10.13mmol) and 10.37g lactide (72mmol) were added to the reaction flask, heated at 70°C to melt the solid, evacuated and filled with argon, repeated the operation three times, removed the water in the reaction solution, added 20mg Stannous octoate solution, continue to vacuum until there are no bubbles in the reaction solution, heat the reaction solution to 180 ° C, react for 6 hours, stop heating, cool to room temperature, add 8 ml of...
preparation example 2
[0115] Preparation Example 2: Examples of other bis-α-cyanoacrylates
[0116] Using a method similar to that in Preparation Example 1 and selecting different intermediate fragments, the following compounds can be synthesized
[0117] Diethylene glycol bis-α-cyanoacrylate
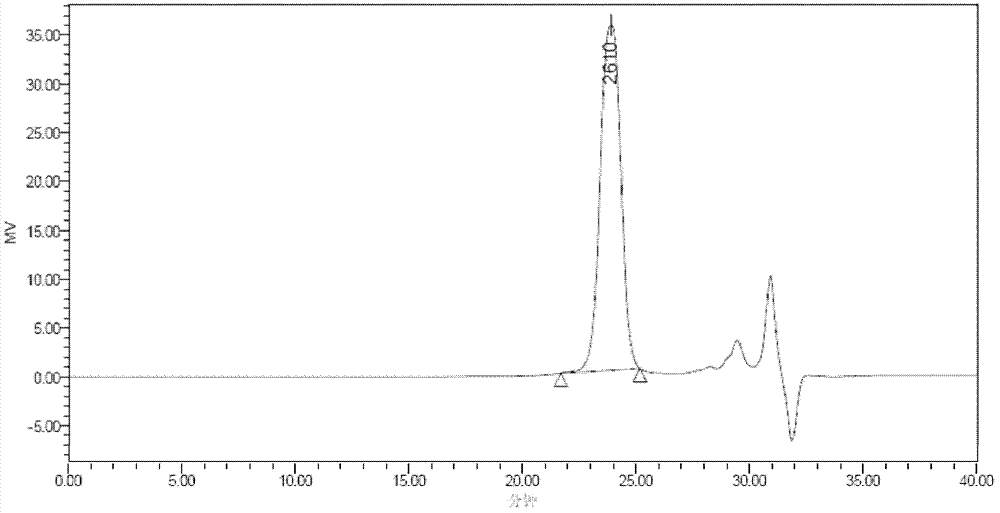
[0118] 1 HNMR (CDCl 3 , δppm): δ7.07(s, 2H), δ6.67(s, 2H), δ4.44(t, 4H), δ3.82(t, 4H) light yellow oily liquid
[0119] Triethylene glycol bis-α-cyanoacrylate
[0120] 1 HNMR (CDCl 3 , δppm): δ7.08(s, 2H), δ6.65(s, 2H), δ4.44(t, 4H), δ3.80(t, 4H), δ3.66-3.70(m, 8H) light yellow oily liquid
[0121] Bisα-Cyanoacrylate PEG600 Ester
[0122] 1 HNMR (CDCl 3 , δppm): δ7.08(s, 2H), δ6.66(s, 2H), δ4.43(m, 4H), δ3.68(m, 54H)
[0123] Bisα-Cyanoacrylate PEG1000 Ester
[0124] 1 HNMR (CDCl 3 , δppm): δ7.08(s, 2H), δ6.71(s, 2H), δ4.42-4.44(m, 4H), δ3.57-3.80(m, 90H) light yellow oily liquid
[0125] Bisα-Cyanoacrylate PEG2000 Ester
[0126] 1 HNMR (CDCl 3 , δppm): δ7.09(s, 2H), δ6.66(s, 2H), δ4.42-4.4...
preparation example 3
[0138] Preparation Example 3: Example of Mono-α-cyanoacrylate
[0139] This kind of compound is carried out with reference to literature reports (Li Jingfeng, Sun Xiping. The preparation technology and application of α-cyanoacrylate adhesive. Colloid and polymer. 1999, 17(3): 33-34; CN 87103468A) general method, namely with Under the condition of basic catalyst, cyanoacetate is polymerized with aqueous formaldehyde solution to form a low molecular weight prepolymer, and then depolymerized and purified at high temperature under reduced pressure. Taking n-butyl cyanoacrylate as an example, its chemical reaction formula is as follows:
[0140]
[0141] Mono-α-cyanoacrylate is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| aperture size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com