Carbazolyl-based organic electroluminescence compound
A compound and luminescent technology, applied in organic chemistry, luminescent materials, circuits, etc., can solve the problems of reduced probability of electron and hole recombination, poor carrier transport performance, and reduced device luminous efficiency, so as to improve luminescence Efficiency and life, satisfying the effect of high preparation efficiency and good electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
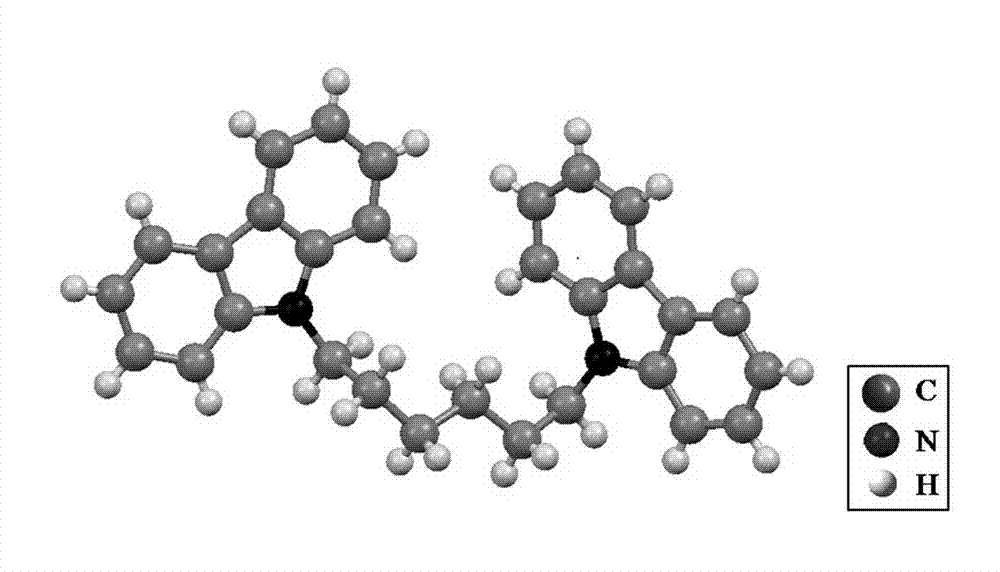
[0045] Dissolve 10.032g of carbazole and 7.3191g of 1,6-dibromohexane in 90ml of tetrahydrofuran in turn, add 0.192g of catalyst tetrabutylammonium bromide under nitrogen protection, stir and mix evenly, add 16mol / L of potassium hydroxide The solution was 40ml, warmed up to reflux, and reacted for 12h. After the reaction, extract the organic layer with dichloromethane, dry over magnesium sulfate, concentrate the solution by rotary evaporation, apply to a silica gel chromatography column, rinse with petroleum ether, evaporate the eluent, and recrystallize and purify with ethanol to obtain white needles 1, 6-dicarbazolylhexane (hCP) crystals.
[0046]
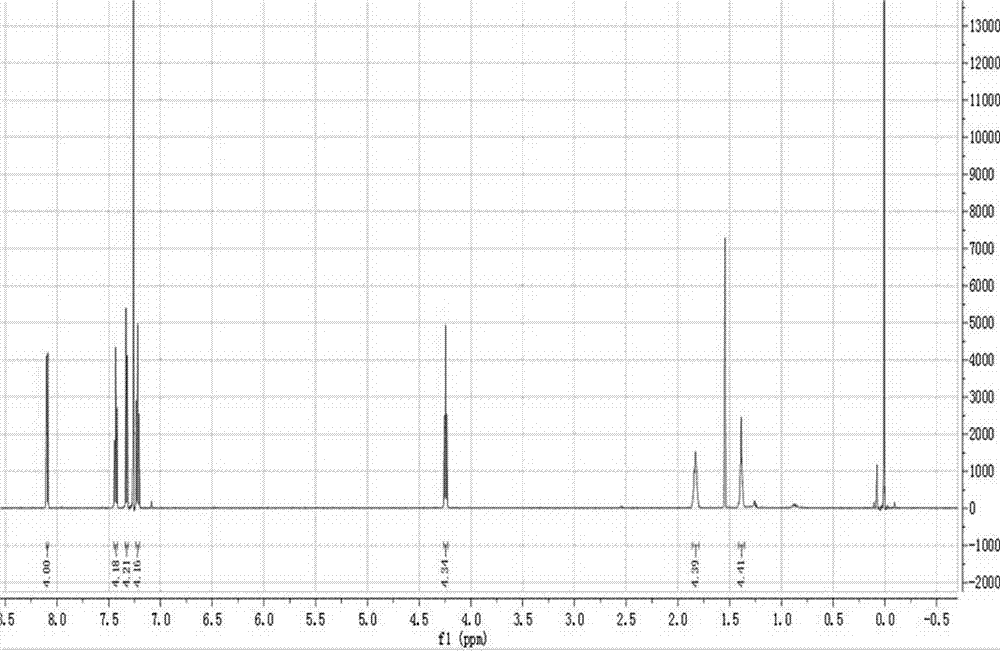
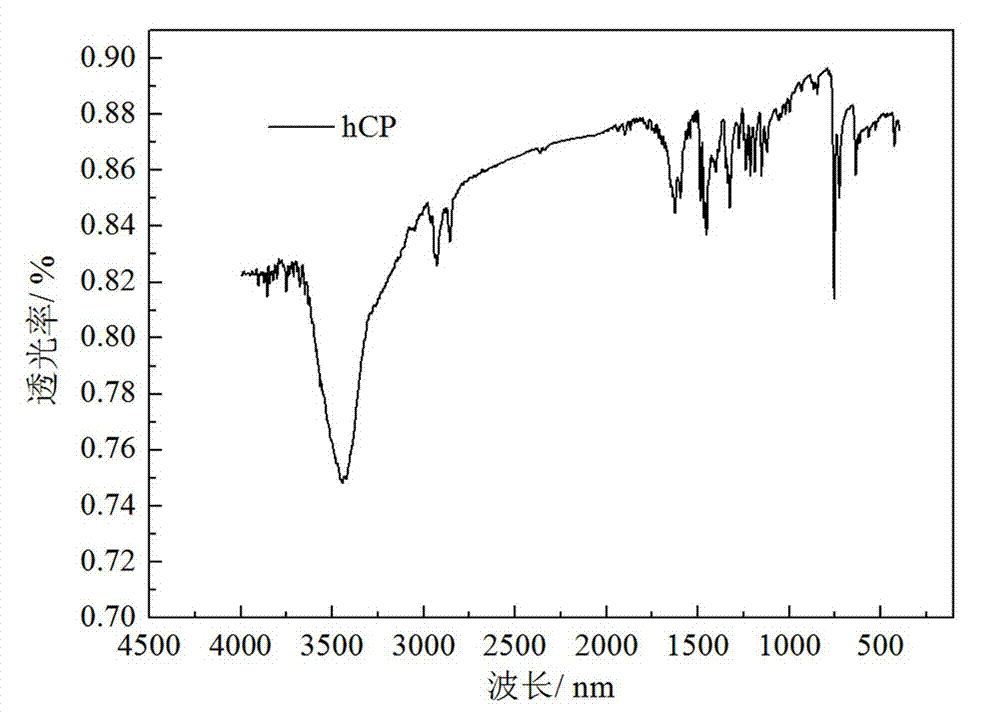
[0047] [0040] by 1 The structures of the obtained compounds were characterized by HNMR and FT-IR, figure 1 middle 1 HNMR (CDCl 3 , 600MHz)δ: 1.3881(m, 4H), 1.8309(m, 4H), 4.2439(td, 4H), 7.2194(ts, 4H), 7.32665(d, 4H), 7.4194(td, 4H), 8.0934(d , 4H); figure 2 Medium FT-IR (KBr): 3445, 2926, 2852, 2360, 1626, 1456, 75...
Embodiment 2
[0065] Dissolve 11.72g of 3,6-dimethylcarbazole and 7.3191g of 1,6-dibromohexane in sequence in 90ml of tetrahydrofuran, add 0.192g of catalyst tetrabutylammonium bromide under nitrogen protection, stir and mix evenly, add 40ml of 16mol / L potassium hydroxide solution was heated to reflux and reacted for 12h. After the reaction, extract the organic layer with dichloromethane, dry over magnesium sulfate, concentrate the solution by rotary evaporation, apply to a silica gel chromatography column, rinse with petroleum ether, evaporate the eluent, and recrystallize and purify with ethanol to obtain white needles 1, 6-bis(4,7-dimethylcarbazolyl)hexane (mhCP) crystals.
[0066]
[0067] The mhCP obtained by the reaction was dissolved in a mixed solution of dichloromethane and petroleum ether with a volume ratio of 1:2, and left for 48 hours to obtain a white needle-like transparent single crystal.
Embodiment 3
[0069] Dissolve 13.39g of 3,6-diethylcarbazole and 7.3191g of 1,6-dibromohexane in sequence in 90ml of tetrahydrofuran, add 0.192g of tetrabutylammonium bromide, stir and mix well, then add 16mol / L of hydrogen Potassium oxide solution 40ml, reflux reaction 12h, above-mentioned operations are all carried out under nitrogen protection. After the reaction, extract the organic layer with dichloromethane, dry over magnesium sulfate, concentrate the solution by rotary evaporation, apply to a silica gel chromatography column, rinse with petroleum ether, evaporate the eluent, and recrystallize and purify with ethanol to obtain white needles 1, 6-bis(4,7-diethylcarbazolyl)hexane (ehCP) crystals.
[0070]
[0071] [0056] The ehCP obtained by the reaction was dissolved in a mixed solution of dichloromethane and petroleum ether with a volume ratio of 1:2, and left for 48 hours to obtain a white needle-like transparent single crystal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| optical band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com