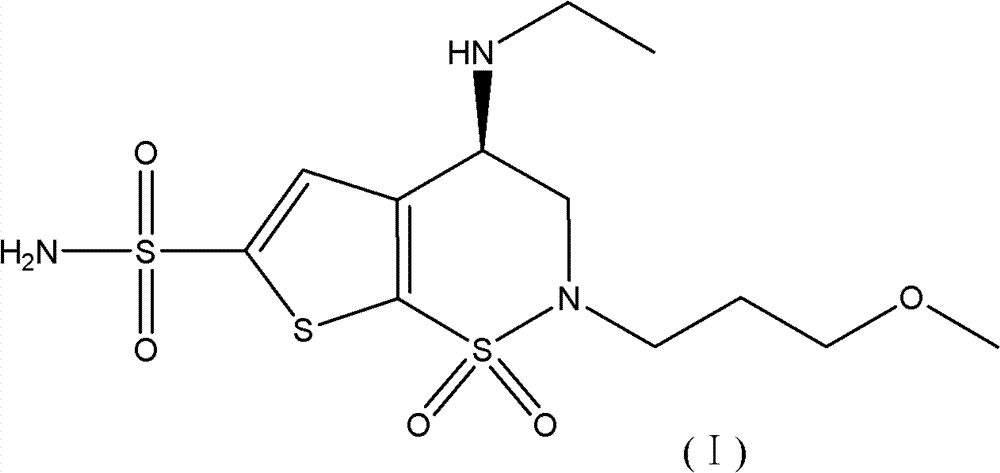
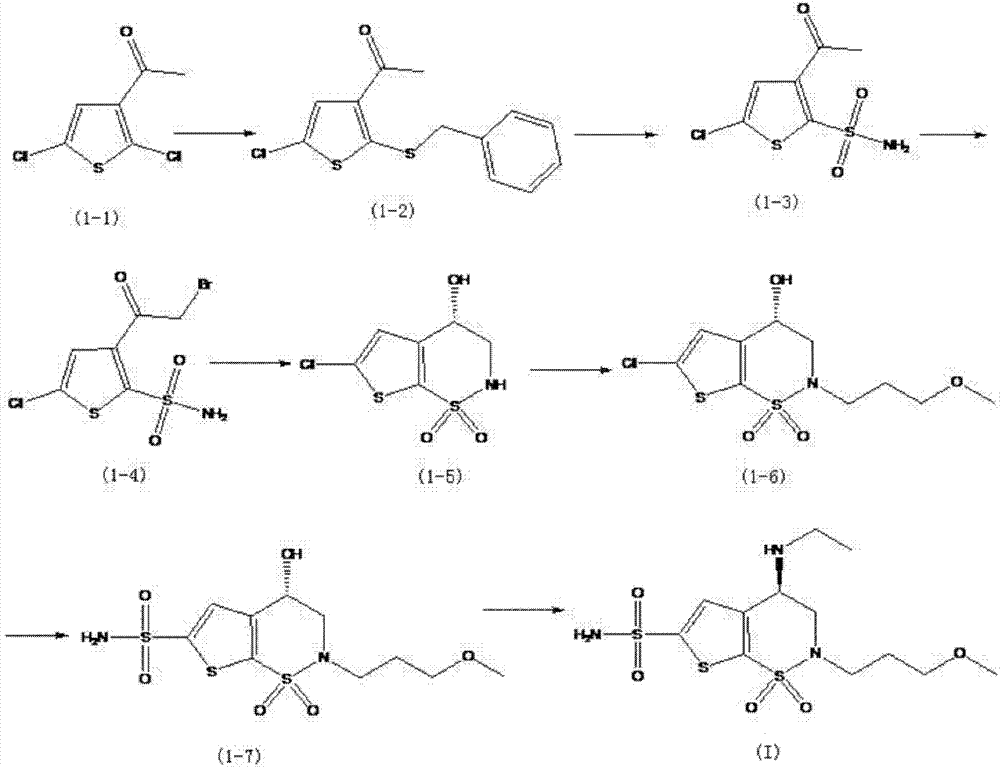
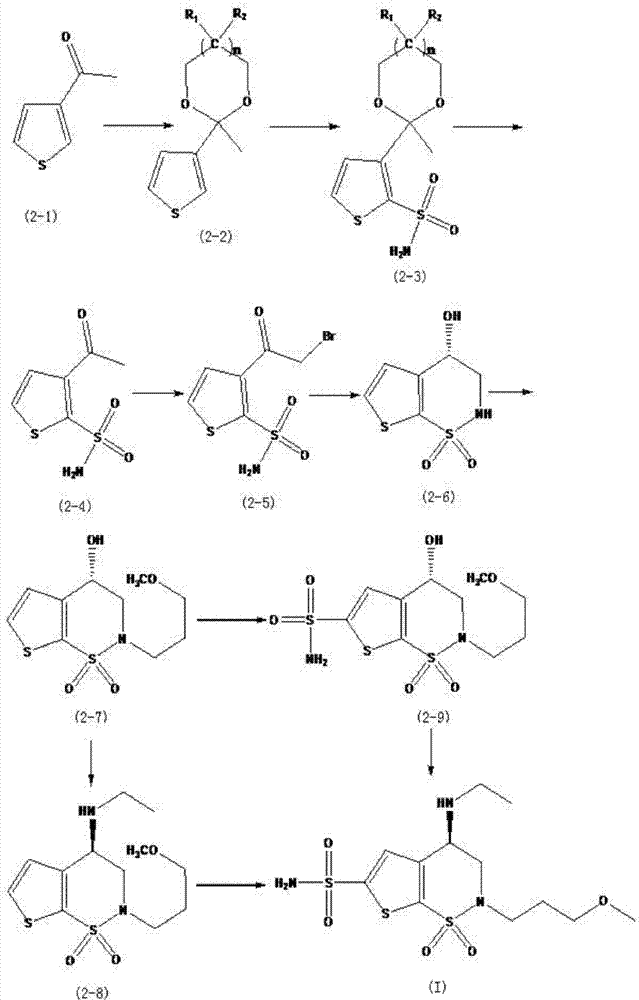
Method for preparation of brinzolamide and intermediates thereof
A compound and amino protecting group technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high price risk, high price of 3-acetylthiophene, difficult production and operation, etc., and achieve low safety risk and cost, Cost-competitive and easy to synthesize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Amination reaction
[0058] Add 1 g of the compound represented by formula (1) and 10 ml of tetrahydrofuran into the reaction vessel, cool down to 0° C., add 0.8 mL of triethylamine, stir overnight at this temperature, and track the reaction result by TLC.
[0059] Cool down to -10°C, add 1 mL of 70% ethylamine aqueous solution, and the reaction is complete at room temperature.
[0060] Cool down in an ice bath, add 11 mL of concentrated hydrochloric acid, control the internal temperature below 20°C, and stir for 1 hour.
[0061] Dichloromethane 20mL×2 extracted, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure.
[0062] Dissolve the oil in 20 mL of ethyl acetate, add 20 mL of n-hexane, stir, and filter to obtain 0.8 g of a pale yellow solid represented by formula (2).
[0063] Mass spectrum (ESI) [M-HCl+1]=375.
[0064] 2. Introducing a protecting group (benzyl)
[0065] Add 0.5 g of the compound represented b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com