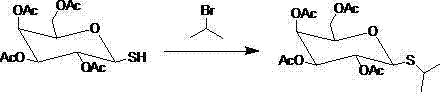
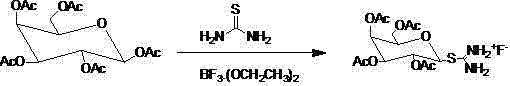
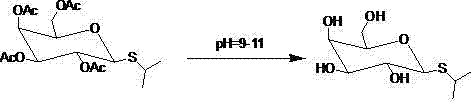Synthetic method for isopropyl-beta-D-thiogalactoside
A technology of thiogalactoside and synthesis method, which is applied in the synthesis field of isopropyl-β-D thiogalactoside, can solve the problems of toxicity, unsuitability for industrial production, low yield and the like, and achieves broad market prospects , easy operation, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] step one:
[0033] Add 23.4g (60mmol) pentaacetylgalactose and 120ml dichloromethane into a 500ml round flask, stir to dissolve, then add 9.12g (120mmol) thiourea to the system, stir thoroughly for 5min, then add boron trifluoride ether Solution 29.4ml (240mmol), stirred at room temperature for twelve hours, a white solid precipitated in the system, followed by TLC to detect the reaction, after the reaction was complete, filtered to obtain a white solid, recrystallized with acetone and petroleum ether to obtain pure S- Tetraacetylgalactose isothiouronium fluoride salt 24.5 grams, the yield is 96%.
[0034] Step two:
[0035] Add 17.1 grams (90 mmol) of sodium metabisulfite, 150 ml of dichloromethane and 150 ml of water into a 500 ml round bottom flask, stir well and add 24.5 grams (57 mmol) of S-tetraacetylgalactose isothiourea fluoride salt, and heat to reflux , reacted for 6-7h, tracked the reaction with TLC (petroleum ether / ethyl acetate=1:1), after the reaction wa...
Embodiment 2
[0041] step one:
[0042] Add 23.4g (60mmol) pentaacetylgalactose and 120ml dichloromethane into a 500ml round flask, stir to dissolve, then add 11.4g (150mmol) thiourea to the system, stir thoroughly for 5min, then add boron trifluoride ether Solution 29.4ml (240mmol), stirred at room temperature for twelve hours, a white solid precipitated in the system, followed by TLC to detect the reaction, after the reaction was complete, filtered to obtain a white solid, recrystallized with acetone and petroleum ether to obtain pure S- Tetraacetylgalactose isothiourea fluoride salt 25.6 grams, yield is 97%.
[0043] Step two:
[0044] Add 19.1 grams (100 mmol) of sodium metabisulfite, 150 ml of dichloromethane and 150 ml of water into a 500 ml round bottom flask, stir well and add 25.6 grams (58 mmol) of S-tetraacetylgalactose isothiuronium fluoride, and heat to reflux , reacted for 6-7h, tracked the reaction with TLC (petroleum ether / ethyl acetate = 1:1), after the reaction was compl...
Embodiment 3
[0050] step one:
[0051] Add 23.4g (60mmol) pentaacetylgalactose and 120ml dichloromethane into a 500ml round flask, stir to dissolve, then add 11.4g (150mmol) thiourea to the system, stir thoroughly for 5min, then add boron trifluoride ether Solution 14.9ml (120mmol), stirred at room temperature for 12 hours, a white solid was precipitated, and the reaction was tracked and detected by TLC. After the reaction was complete, the white solid was obtained by filtration and recrystallized to obtain pure S-tetraacetylgalactose isothio Urea fluoride salt 19.0 grams, yield is 74%.
[0052] Step two:
[0053] Add 10.0 grams (50 mmol) of sodium metabisulfite, 150 ml of dichloromethane and 150 ml of water into a 500 ml round bottom flask, stir well and add 19.0 grams (44 mmol) of S-tetraacetylgalactose isothiuronium fluoride salt, and heat to reflux , reacted for 6-7h, tracked the reaction with TLC (petroleum ether / ethyl acetate = 1:1), after the reaction was completed, cooled to room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com