Preparation and application of tumor marker calreticulin detection kit
A calreticulin and kit technology, applied in the fields of genetic engineering and clinical diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
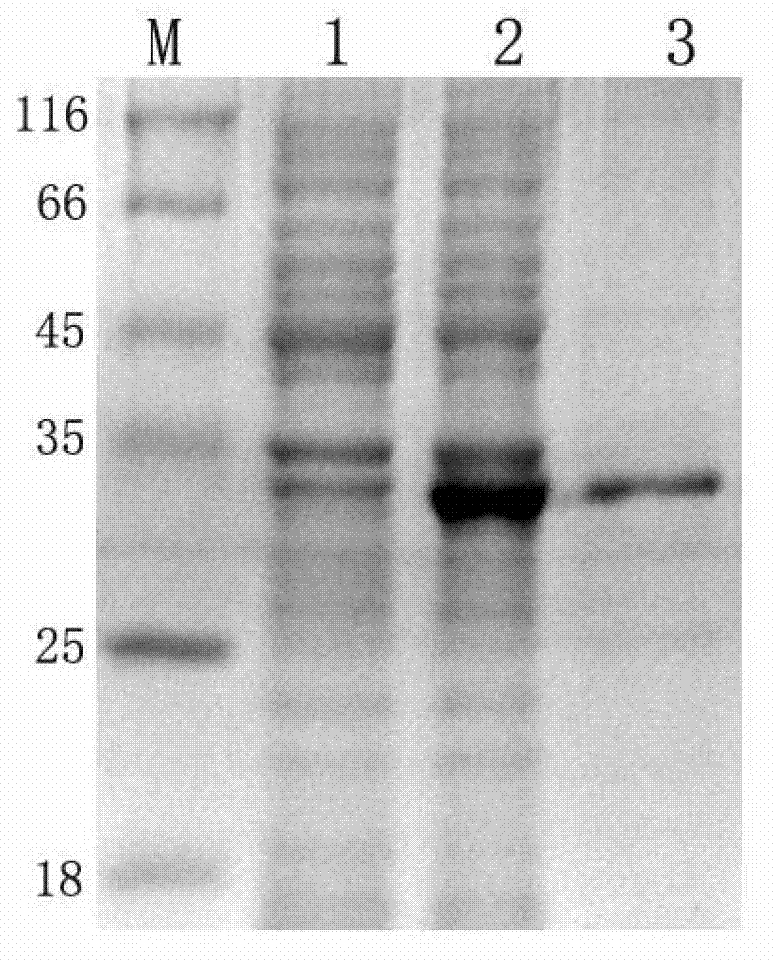
[0044] Embodiment 1, preparation and identification of CRT monoclonal antibody
[0045] 1. Preparation of monoclonal antibody
[0046] Before immunizing Balb / c mice, about 100 μl of serum was obtained and stored at -20°C, and then 50 μg of purified CRT antigen was mixed with Freund’s complete adjuvant to make an emulsion, and then immunized by multi-point injection in the back of the skin. One month later, 25 μg of the antigen plus Freund's incomplete adjuvant was injected intradermally for booster immunization three times with an interval of 2 weeks each time. After the titer test reaches the standard, the mouse splenocytes obtained by immunization are fused with SP2 / 0 myeloma cells, the feeder layer cells are mouse peritoneal macrophages, and the fusion reagent is 50% PEG4000, splenocytes and myeloma cells The ratio of cells was 5:1, placed in 5% CO 2 Cultivate in a 37°C incubator with saturated humidity. On the 3rd, 6th, 9th, and 10th day, change into the complete 1640 ...
Embodiment 2
[0059] Example 2, Preparation and Identification of CRT Polyclonal Antibody
[0060] 1. Preparation of multiple antibodies
[0061] First obtain about 5ml serum of New Zealand white rabbits before antigen injection, and then mix 500μg CRT antigen with Freund's complete adjuvant to make emulsion, and inject it into the back of the skin at multiple points for immunization. One month later, 250 μg of the antigen plus Freund's incomplete adjuvant was injected intradermally for 3 booster immunizations, with an interval of 2 weeks each time. After the titer was determined, blood was collected from the carotid artery and centrifuged to collect serum, purified by saturated ammonium sulfate precipitation, and then added with an equal volume of glycerol to store at -20°C.
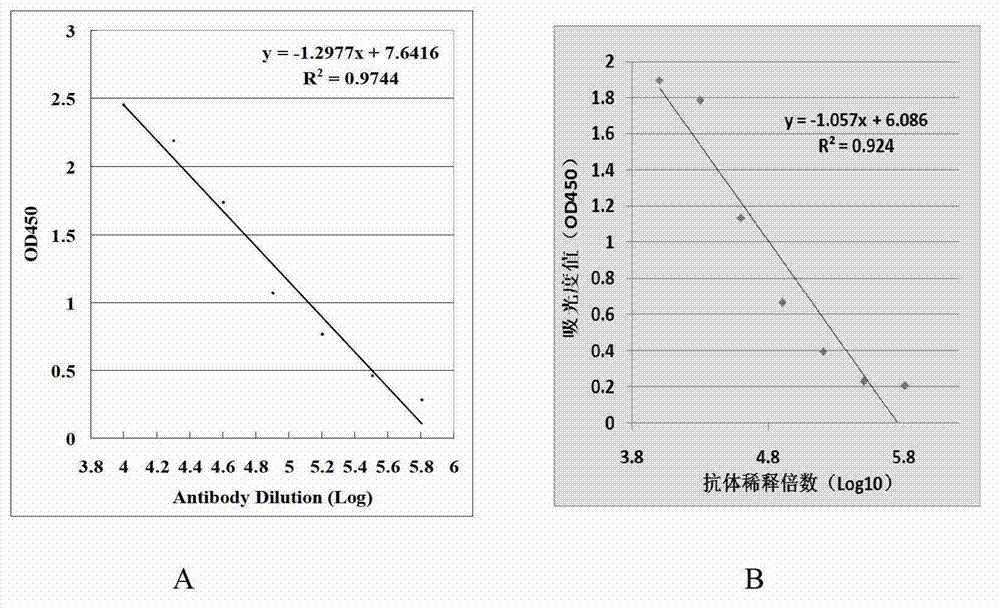
[0062] 2. Determination of antibody titer by ELISA method
[0063] Coat CRT antigen (concentration: 1 μg / ml, 100 μl per well) with carbonate buffer solution for 16 hours at 4°C;
[0064] Wash the ELISA plate onc...
Embodiment 3
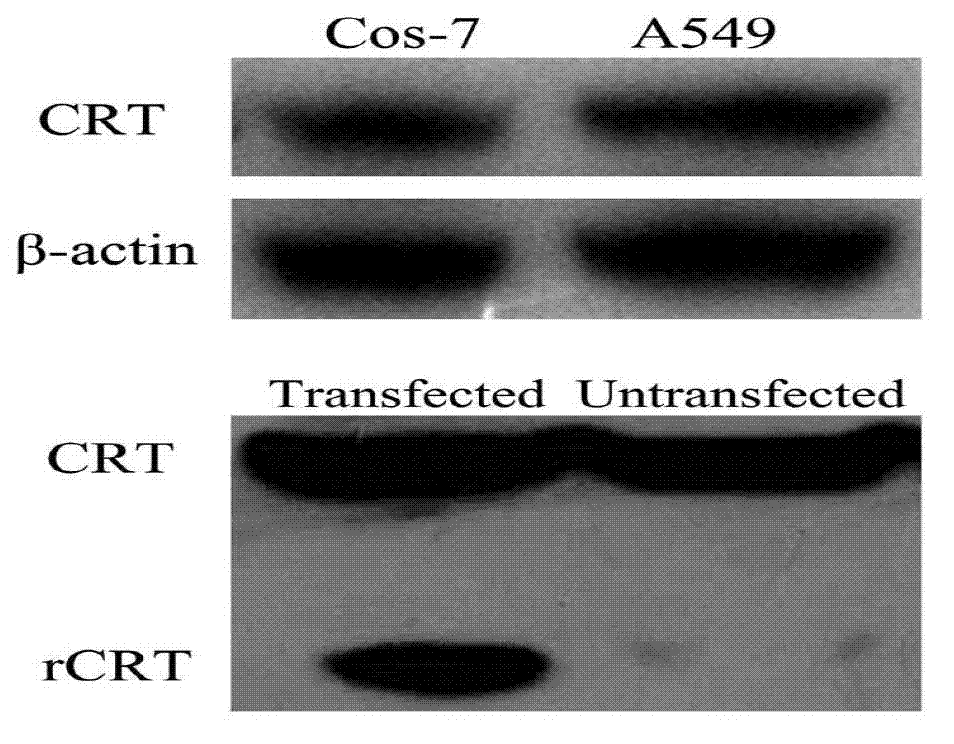
[0073] Embodiment 3, establishment of double-antibody sandwich ELISA detection method
[0074] 1. Basic steps of double-antibody sandwich ELISA assay
[0075] With carbonate buffer (0.05MNa 2 CO 3 / NaHCO 3 , pH9.6)) was coated with 1:800 CRT monoclonal antibody, 100 μl per well was coated at 4°C for 16 hours, and washed once with PBST;
[0076] Add 200 μl blocking solution (1% BSA and 1% goat serum PBS) to block at 37°C for 1 hour, and wash once;
[0077] Add 100 μl of CRT antigen diluted with 1% BSA (0.5 μg / ml, 100 μl / well) at 37°C for 1 hour, and wash 3 times with PBST;
[0078] Add 100 μl CRT polyclonal antibody (4500×), incubate at 37°C for 1 hour, then wash with PBST 3 times;
[0079] Add 100 μl of HRP-labeled goat anti-rabbit antibody (3000×) and incubate at 37°C for 1 hour, wash with PBST 3 times;
[0080] 100μl / well A+B color developing solution was developed for 15 minutes at room temperature in the dark;
[0081] Add 50μl / well of 2mol / LH 2 SO 4 The reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com