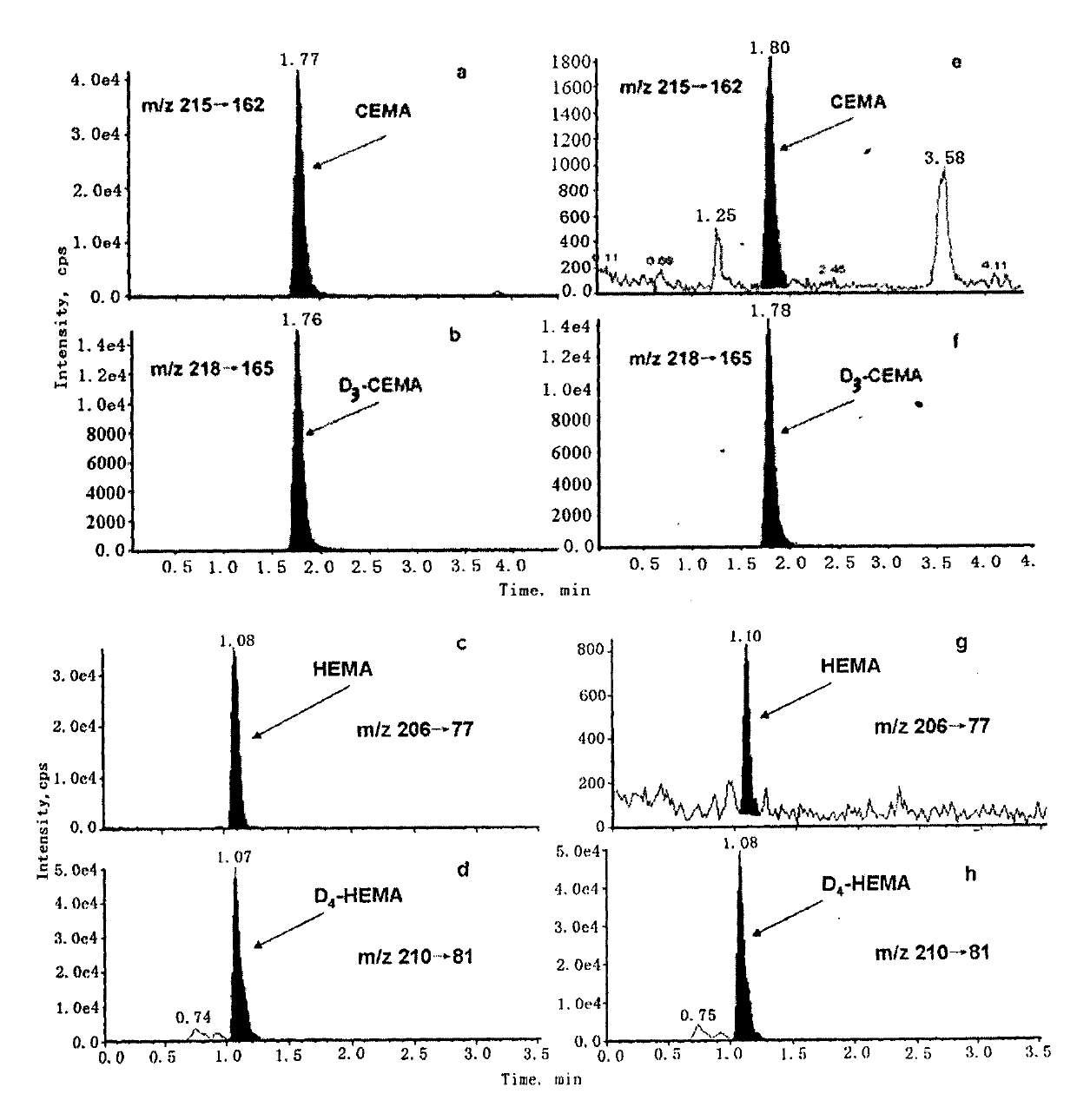
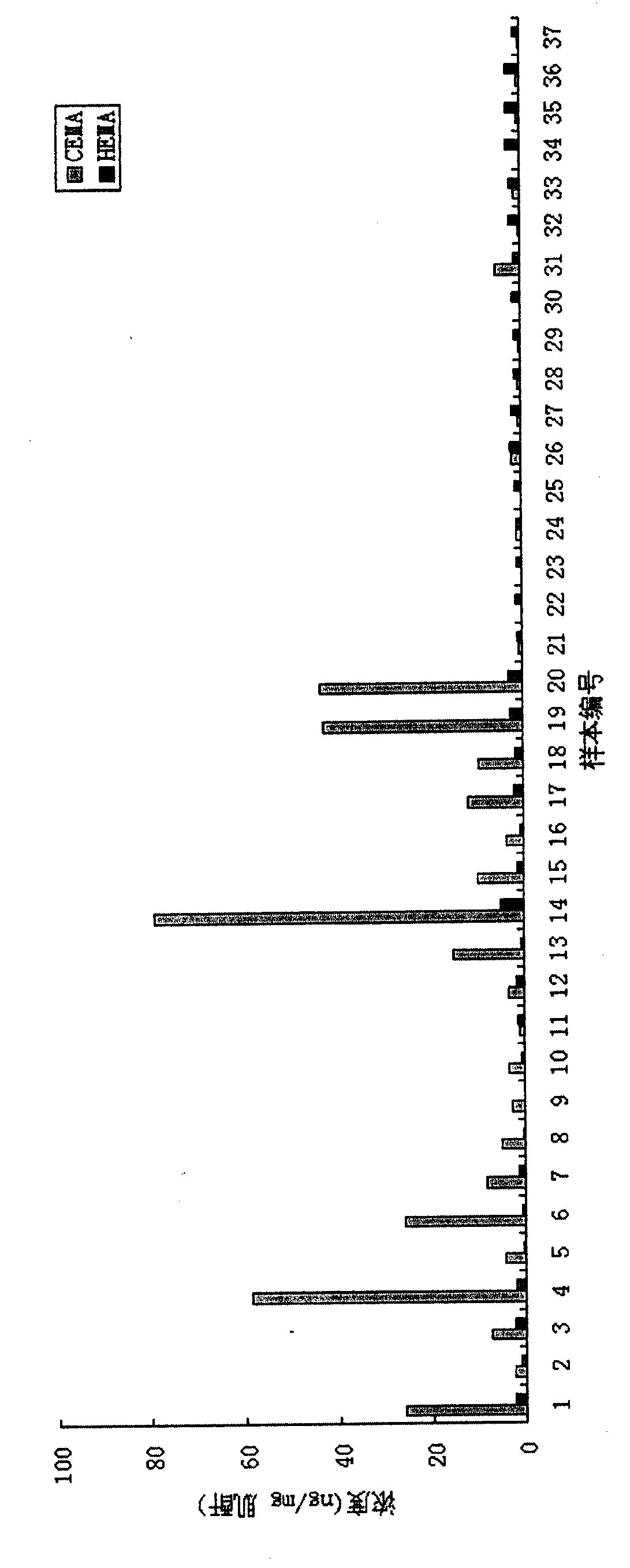
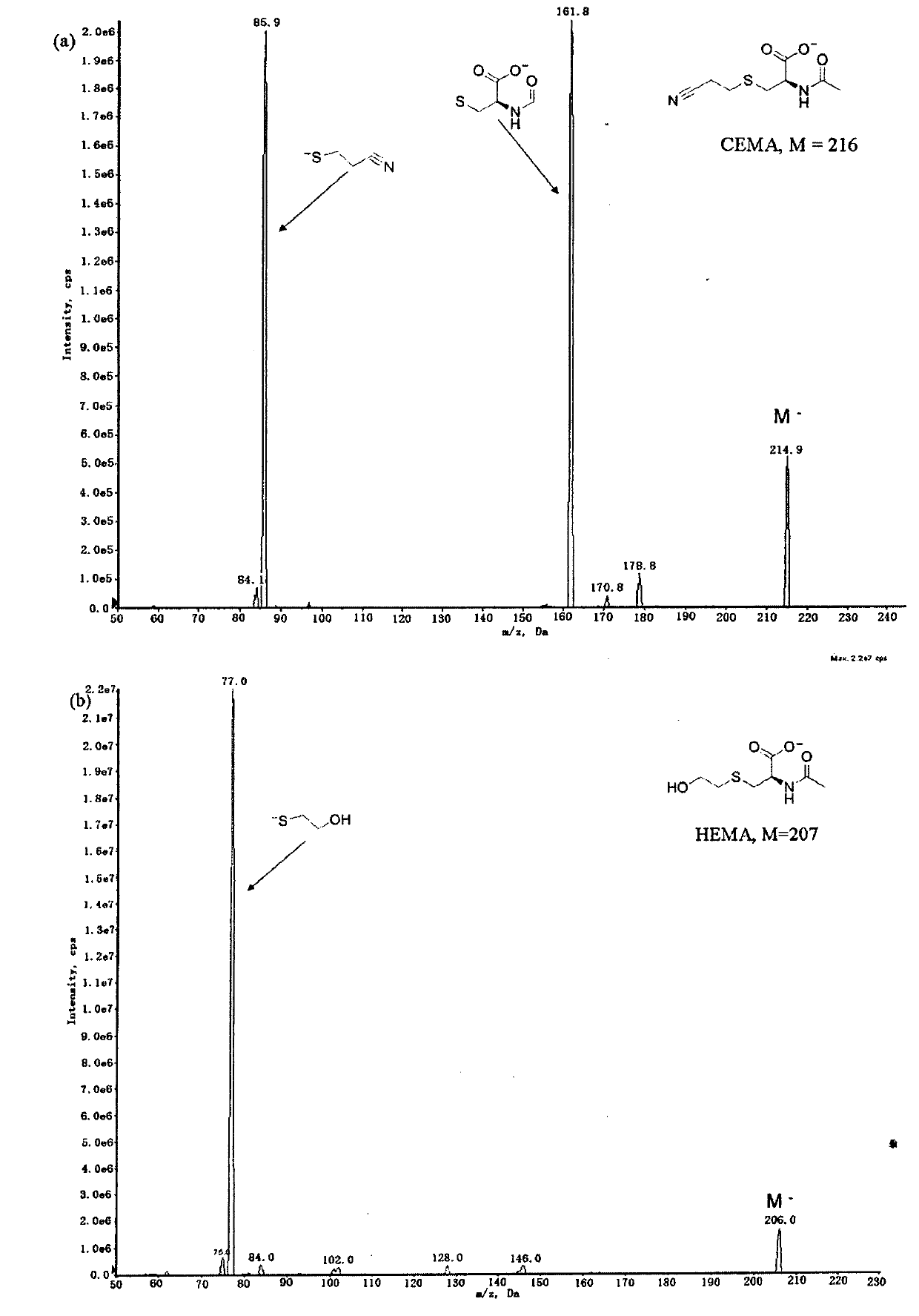
Liquid-chromatogram and tandem-mass-spectrogram assay method for CEMA (Cyanoethyl Methacrylate) and HEMA (Hydroxyethyl Methacrylate) in urine
A measurement method, tandem mass spectrometry technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve good sensitivity and repeatability, reduce errors, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] 1. Instruments and reagents: Agilent 1200 fast high-resolution liquid chromatography (Agilent, USA), equipped with G1367D autosampler, G1312B binary mixing pump and G1316B column thermostat; API 5500 triple quadrupole tandem mass spectrometer (Applied Biotechnology, USA) Systems Company), equipped with electrospray ion source and analyst 1.5 software data processing system.
[0049] N-acetyl-S-(2-cyanoethyl)-L-cysteine (purity 98%), N-acetyl-S-(2-hydroxyethyl)-L-cysteine ( Purity 98%), N-acetyl-S-(2-cyanoethyl)-L-cysteine-D3 (chemical purity 98%, isotopic purity 99%), N-acetyl-S-(2 -Hydroxyethyl)-L-cysteine-D4 (chemical purity 98%, isotopic purity 99%) (Toronto Research Chemicals). Formic acid (chromatographically pure, Acros Organics). Methanol and acetonitrile (chromatographically pure, TEDIA company).
[0050] 2. Sample handling: Thaw urine at room temperature and mix well. Accurately pipette 1.0 mL of urine, add 10 μL of formic acid to acidify, and centrif...
example 2
[0052] Example 2: As described in Example 1, 37 urine samples were taken. The content of CEMA and HEMA in urine was measured as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com