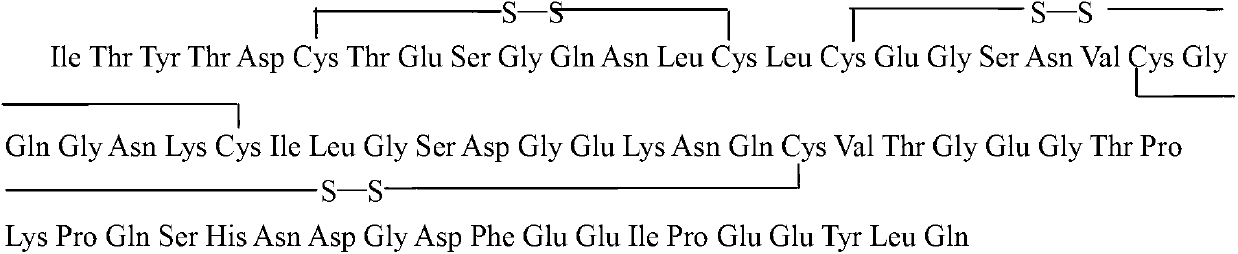
Hirulog as well as preparation method and application thereof
A technology of leech peptide and recombinant hirudin, which is applied to the preparation method of peptides, from leech inhibitors, chemical instruments and methods, etc., can solve the problems of small immunogenicity, affecting use, haptenism, etc., and achieve immunogenicity The effect of reducing, good development prospects and clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0025] (1) 100 rats were injected with recombinant hirudin into the tail vein at a dose of 6 mg / kg. Collect urine 0.5h after administration for the separation and extraction of metabolites;
[0026] (2) Preliminary separation of blank and sample urine by gel chromatography. Chromatographic conditions: gel column, TSK-GELG2000SW 7.5mm×30.0cm, injection volume: 20μl, mobile phase: 0.02mol / l PBS solution, pH7.0, flow rate 0.8ml / min, detection wavelength: 215nm. Collect the effluent, collecting 1 tube per minute. Take the collected solution at 16, 17, 20, and 21 minutes, freeze at -70°C, freeze-dry with a freeze dryer the next day, and set aside;
[0027] (3) The 16-21 min collected liquid collected by gel chromatography was separated again by C8 reverse phase chromatography. Chromatographic conditions: C8 column, injection volume 20μl, gradient elution, acetonitrile: 0.1% TFA from 15:85 to 45:55 within 25min, flow rate 0.5ml / min, detection wavelength 215nm. Inject samples rep...
Embodiment 3
[0029] Determination of molecular weight
[0030] The above-mentioned leech peptide rH 1-51 and rH 1-52 , the molecular weight of the extracted metabolites was determined by time-of-flight mass spectrometry (MALDI-TOF / TOF). Detection conditions: N2 laser source, wavelength 337nm; detection method: positive ion, linear method (flight tube length 1.5m, acceleration voltage 20kV); matrix: CCA, leech peptide rH 1-51 and rH 1-52 The molecular weight was determined to be 5162 by time-of-flight mass spectrometry.
Embodiment 4
[0032] Determination of antithrombin activity
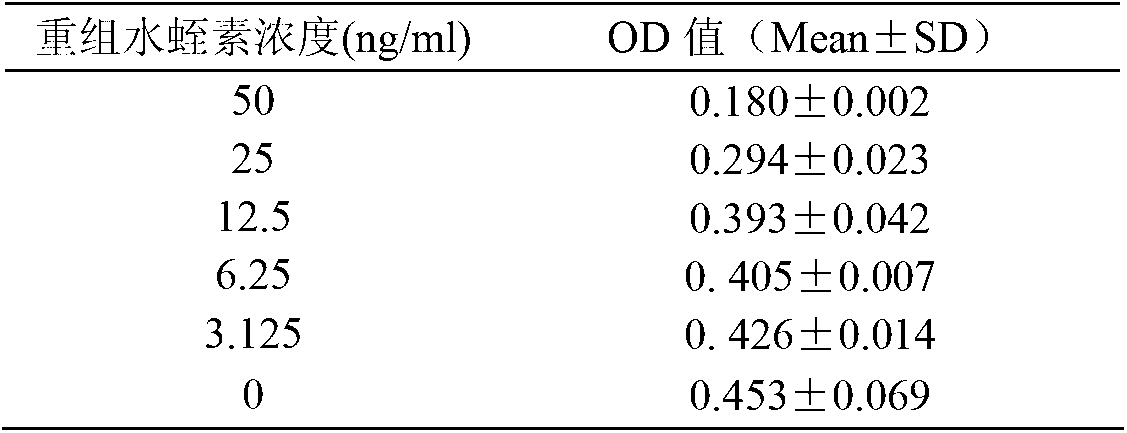
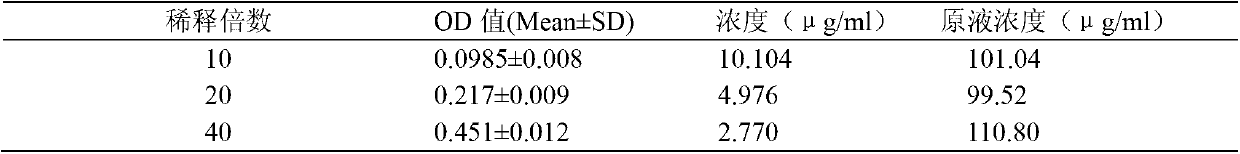
[0033] This method uses tosyl-aminoacetyl-prolyl-arginyl-p-nitroaniline (Tosyl-glycyl-polyl-arginine-para-nitroniline; trade name: Chromozym TH) as the substrate, thrombin It can hydrolyze Chromozym TH to release p-nitroaniline (para-nitroniline, pNA), and pNA has specific absorption at 405nm. rH can be quantitatively combined with thrombin, so that thrombin loses the activity of hydrolyzing the substrate. By measuring the amount of thrombin not inhibited by rH in the reaction system, the content of rH can be indirectly measured. In this experiment, rH was used as a standard to prepare a standard curve, and the rH 1-51 and rH 1-52 Antithrombin activity.
[0034] 1. Preparation of standard curve
[0035] Recombinant hirudin is a specific inhibitor of thrombin. Thrombin can hydrolyze the chromogenic substrate (Chromozym TH, CT) to release p-nitroaniline, which has absorption at 405nm wavelength, within a certain range , the am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com