Method for producing high-purity vanadium pentoxide by chlorination
A technology of vanadium pentoxide and production method, applied in vanadium oxide and other directions, to achieve the effects of saving water resources, avoiding pollution, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
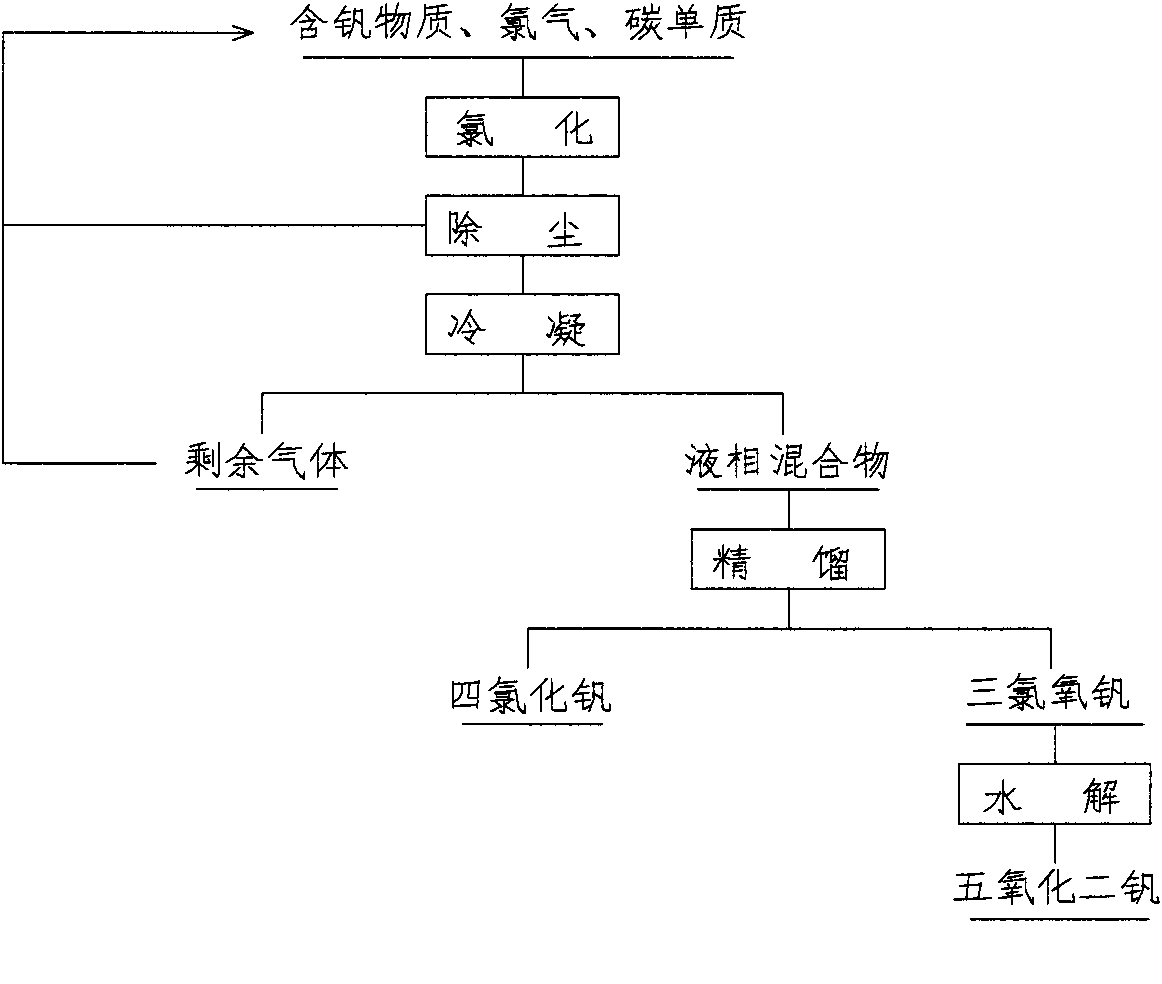
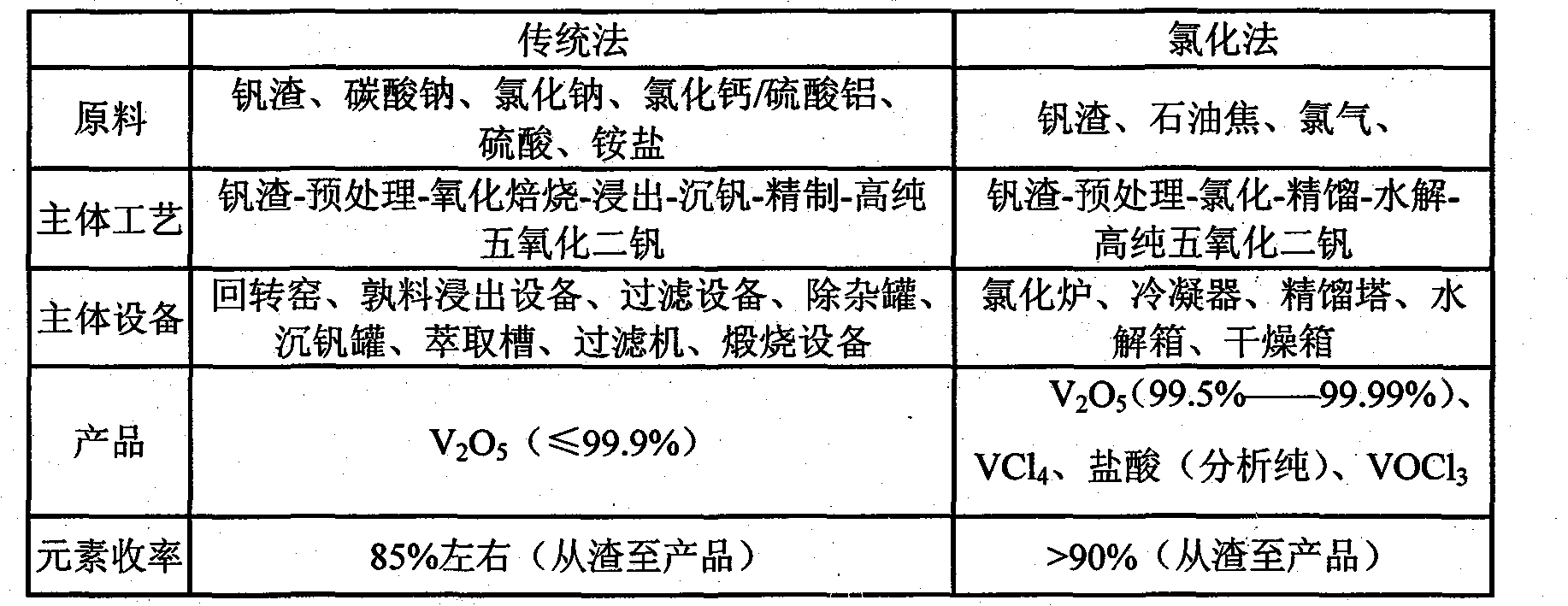
[0013] 50gV 2 o 5 (98%) mixed evenly with 16g graphite powder, put it into the back graphite crucible, and then dry it in the oven at 120°C for two hours, put it into the boiling chlorination furnace, feed dry nitrogen, heat up to 350°C and keep it warm. Gradually reduce the nitrogen feed rate and slowly feed dry chlorine gas, and finally replace the nitrogen gas completely with chlorine gas, and the reaction time is 60 minutes. The gaseous mixture generated by the reaction is passed through the cyclone dust collector, and the dust collector is mainly carbon particles and V that have not participated in the reaction. 2 o 5 Solid particles, after treatment, can be returned to the raw material area for further use. The obtained gaseous mixture passes through the condensing device to obtain the liquid phase mixture of vanadium chloride, and the uncondensed gaseous substances can be recycled after being purified. Pass the obtained liquid phase mixture through the secondary rec...
example 2
[0015] 100g vanadium slag (containing V 2 o 5 About 16%), 25g graphite powder, 350gKCl and 150gNaCl are mixed evenly and then put into an oven and dry at 120°C for two hours, then place in a molten salt chlorination furnace and heat up to 600°C-700°C. Inject dry nitrogen, and when the temperature reaches 600°C, reduce the amount of nitrogen introduced and gradually increase the amount of chlorine until it is at the same rate as chlorine. Control the temperature in the range of 650°C-700°C for 120min. Pass the generated gaseous mixture through a condensing device to obtain a liquid phase mixture of vanadium chloride, and the uncondensed gaseous substances can be recycled after being purified. Pass the obtained liquid phase mixture through the secondary rectification device to obtain vanadium oxytrichloride and vanadium tetrachloride liquid respectively, and vanadium tetrachloride is properly stored in a special container. Slowly pour the vanadium oxychloride liquid into the ...
example 3
[0017] 100g vanadium slag (containing V 2 o 5 about 16%), 25g of coke powder is mixed evenly, put into graphite crucible and then dry at 120°C for two hours, then place in shaft furnace, heat up to 600°C under the protection of dry nitrogen, then start to introduce chlorine gas and Reduce the feed amount of nitrogen to 0, and control the temperature within the range of 630°C-670°C for 150min. The following steps are the same as Example 2. The obtained vanadium pentoxide has a purity of 99.70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com