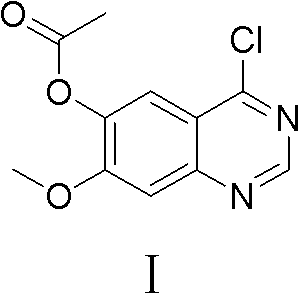
Preparation method of 4-chloro-7-methoxyl quinazoline-6-alchol acetate
A technology of methoxyquinazoline and alcohol acetate, applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of accelerated product conversion, low yield, low product purity, etc., and achieves reduction of the generation of reaction impurities and simple operation. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
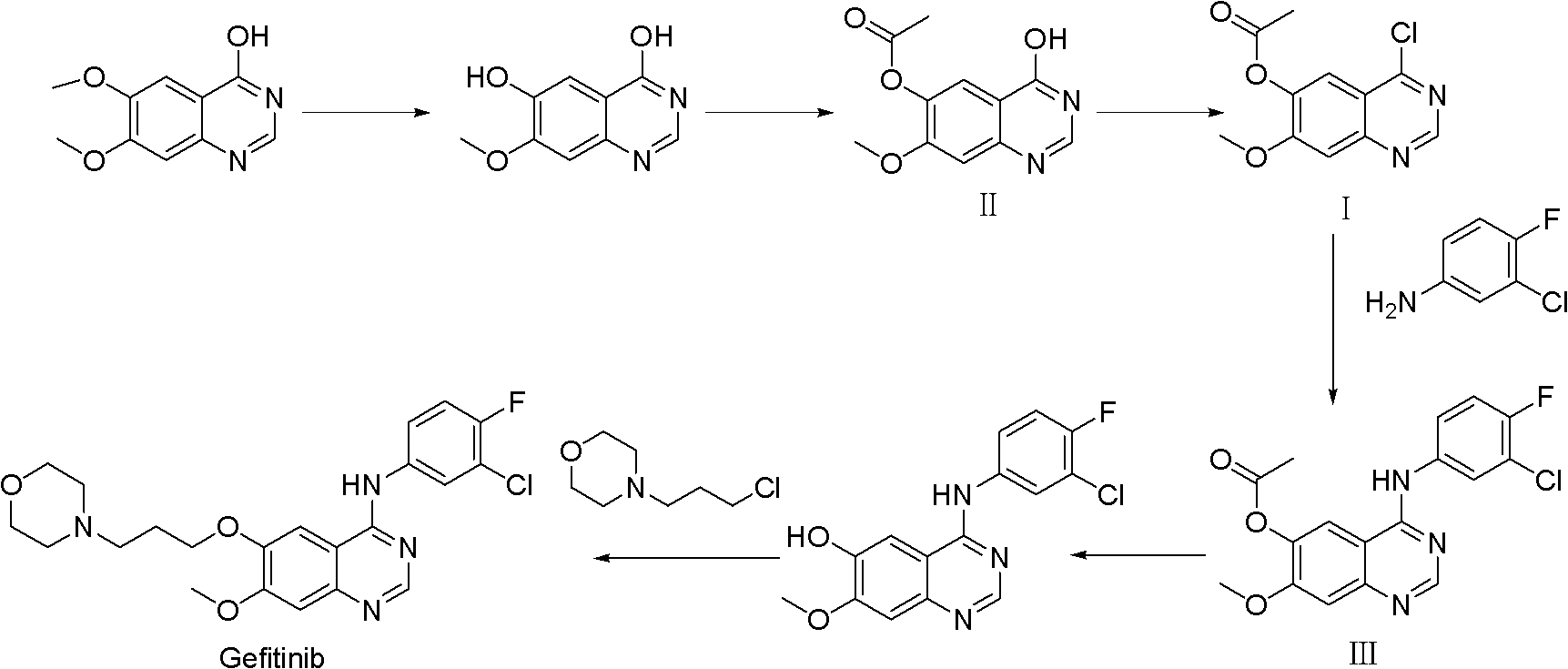
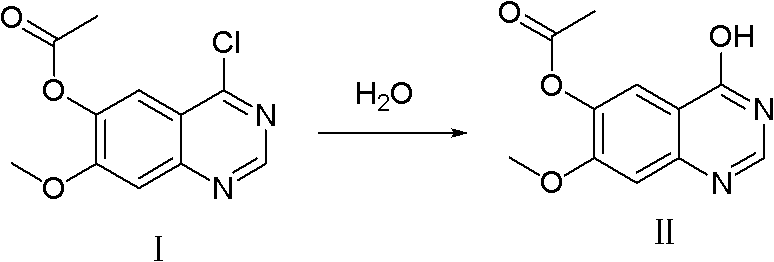
[0038]Add 400L of dichloromethane into the reaction kettle, add 3,4-dihydro-7-methoxy-4-oxoquinazolin-6-alcohol acetate 30kg (compound II, 128mol) under stirring, add Thionyl chloride 60kg (504mol), N,N-dimethylformamide 3L; after the addition was completed, the temperature was raised to reflux for 3 hours, the reaction system was lowered to room temperature, and sodium bicarbonate solid was slowly added to adjust the pH to 6.5, centrifuged, and reduced Evaporate to dryness to obtain 4-chloro-7-methoxyquinazolin-6-ol acetate (compound I) as a yellow solid with a purity of 98.21%, which can be used in subsequent reactions without purification.
[0039] Compound I obtained by the above method was added to 150L of isopropanol, 20.0 kg of 3-chloro-4-fluoroaniline was added, reacted at room temperature for 1 hour, and suction filtered; dried to obtain 4-(3-chloro-4-fluoroaniline)- 45.6 kg of 7-methoxyquinazolin-6-ol acetate (compound III), purity 98.36%, two-step yield: 96.5%.
Embodiment 2
[0041] 350ml of chloroform was added to the reaction flask, and 3,4-dihydro-7-methoxyl-4-oxoquinazolin-6-alcohol acetate 30.0g (compound II, 0.128mol ), add 180g (1.174mol) of phosphorus oxychloride, 5ml of N,N-dimethylformamide; after the addition is completed, the temperature is raised to reflux for 6h, the reaction system is lowered to room temperature, and sodium carbonate solid is slowly added to adjust the pH=8. After centrifugation and reduced evaporation to dryness, 32.3 g of 4-chloro-7-methoxyquinazolin-6-ol acetate (Compound I) was obtained as a yellow solid with a purity of 97.52% and a yield of 99.8%.
Embodiment 3
[0043] Add 400ml of toluene to the reaction flask, add 3,4-dihydro-7-methoxy-4-oxoquinazolin-6-alcohol acetate 30.0g (compound II, 0.128mol) under stirring, add 100g (0.788mol) of oxalyl chloride, 3ml of N,N-dimethylformamide; after the addition, raise the temperature and reflux for 5 hours, lower the reaction system to room temperature, slowly add solid ammonium bicarbonate, adjust the pH to 6, centrifuge, and evaporate To dryness, 32.3 g of 4-chloro-7-methoxyquinazolin-6-ol acetate (compound I) was obtained as a yellow solid with a purity of 98.16% and a yield of 99.5%.
[0044] Add compound I obtained by the above method to 150ml of isoamyl alcohol, add 23.0g of 3-chloro-4-fluoroaniline, react at room temperature for 1h, and filter with suction; dry to obtain 4-(3-chloro-4-fluoroaniline)- 44.8 g of 7-methoxyquinazolin-6-ol acetate (compound III), purity 98.25%, two-step yield: 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com