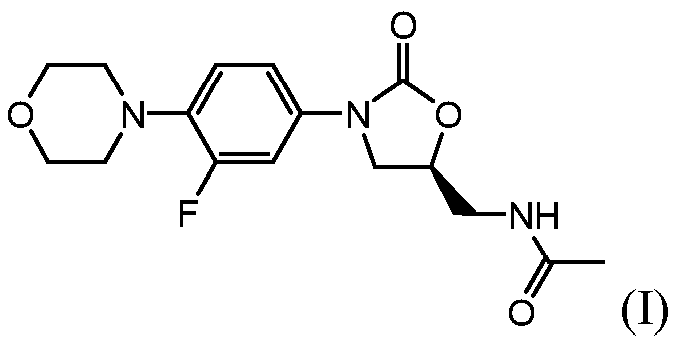
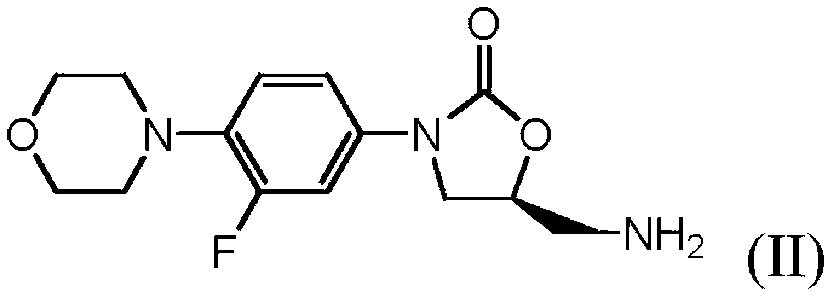
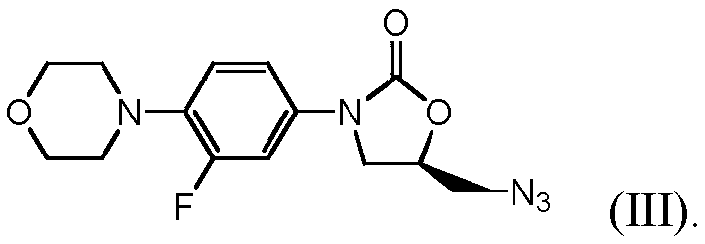
Process for making linezolid
A technology of linezolid and oxazolidine, which is applied in the field of preparing compound linezolid, and can solve the problems of low yield, explosion hazard, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The raw material in the method for preparing linezolid according to the present invention is a compound of general formula (VII)
[0042]
[0043] wherein L is a leaving group, typically a halo group or an alkylsulfonyloxy or arylsulfonyloxy group. A "halo" group includes a chloro or bromo group, preferably a chloro group. "Alkyl" includes C 1 -C 4 Alkyl, and preferably methyl. "Aryl" includes phenyl, which may optionally be replaced by at least one C 1 -C 4 Alkyl, nitro, hydroxyl, C 1 -C 4 Alkoxy substituted, and preferably p-tolyl or p-nitrophenyl.
[0044] Said compounds are known in the art or can be prepared according to methods known in the art, for example from the corresponding hydroxy-methyl compound of formula (X).
[0045]
[0046] The second reaction accompaniment is a metal salt of a diformylamide of formula (VIII).
[0047]
[0048] "Me + " is typically sodium or potassium, preferably sodium. Compound (VIII) is known in the art and can g...
Embodiment 1
[0063] Example 1 3-(3-fluoro-4-(morpholin-4-yl)phenyl)-2-oxo Oxazolidine-5(S)-ylmethyl)amine (II) hydrochloride
[0064] To a 5ml reaction flask equipped with a magnetic stirring bar was added 225mg (0.5mmol) of compound (VII) [L = p-toluenesulfonyloxy], followed by 96mg (1mmol) of sodium diformylamide and 2.5ml of dimethylformamide. The vessel was immersed in an oil bath preheated to 85°C and the mixture was stirred for 1 hour at an external temperature of 85°C. The volatiles were evaporated at reduced temperature (30 mbar, 60° C.), the residue was mixed with 10 ml of ethyl acetate and extracted with 10 ml of water. The organic layer was evaporated to dryness (45 °C, 25 mbar) to give a white solid.
[0065] The solid was mixed with 10 ml of methanol and 0.438 ml of concentrated hydrochloric acid (5.00 mmol) was added. The reaction mixture was warmed to 65°C in an oil bath and stirred at this temperature for 2 hours. The mixture was evaporated under reduced pressure and...
Embodiment 2
[0066] Example 2 3-(3-fluoro-4-(morpholin-4-yl)phenyl)-2-oxo Oxazolidine-5(S)-ylmethyl)amine (II)
[0067] A 250ml three-necked round-bottomed flask equipped with a paddle mechanical stirrer and an Ar inlet combined with a temperature sensor was placed under an argon atmosphere and added sodium diformylamide (5.6g) and compound (VII) [L = p-toluene Sulfonyloxy] (20.8 g). N,N-Dimethylformamide (52ml) was added and the reaction mixture was heated to 85°C for 2 hours. Hydrochloric acid (98ml) was carefully added to the reaction mixture so that the internal temperature did not exceed 96°C. The heterogeneous reaction mixture was heated to 85°C and the reaction was continued for 2.5 hours. The reaction mixture was transferred to a 500ml round bottom flask and cooled in an ice water bath. Dichloromethane (150ml) was added to the flask. The mixture was stirred vigorously and a solution of NaOH (54.0 g) in water (100 ml) was added such that the internal temperature did not exce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com