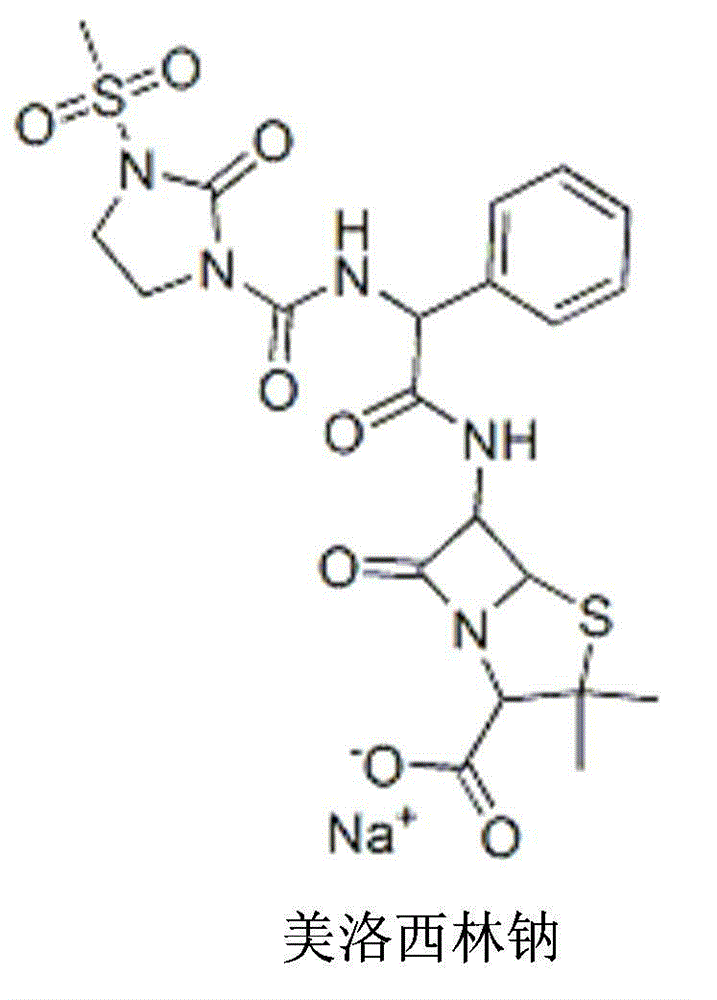
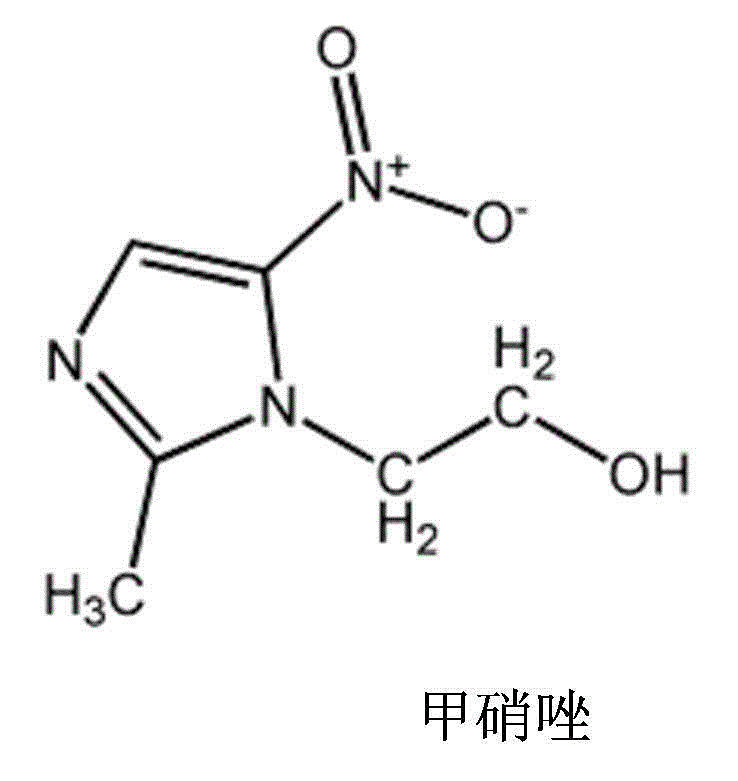
Mezlocillin sodium pharmaceutical composition for injection and preparation method thereof
A technology of mezlocillin sodium and its composition, which is applied in the field of mezlocillin sodium pharmaceutical composition for injection and its preparation, can solve the problems of strong toxic and side effects of metronidazole, large amount of metronidazole, etc., and achieve simple preparation process, The effect of ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, mezlocillin sodium composition freeze-dried powder for injection, in 1000 pieces
[0032] Prescription (specification: 0.5g / bottle):
[0033]
[0034] Preparation Process:
[0035] Add the prescribed amount of mezlocillin sodium and the prescribed amount of metronidazole lipid microspheres (calculated as metronidazole) directly to the water for injection (theoretical loading is 2ml / cartridge); stir and dissolve and then add the pH regulator Adjust the pH value of the solution so that it is in the range of 6.5-6.8; then pass through 0.45μm and 0.22μm filter membranes for sterilizing and circulating filtration for 30 minutes; after sampling and inspection meet the requirements, fill to determine the filling volume, and the second half of the filling volume is qualified Fasten the butyl rubber stopper for freeze-drying and enter the freeze-drying box for freeze-drying treatment to obtain the freeze-dried powder injection of the mezlocillin sodium composit...
Embodiment 2
[0036] Embodiment two, mezlocillin sodium composition freeze-dried powder for injection, in 1000
[0037] Prescription (specification: 1.0g / bottle):
[0038]
[0039]
[0040] Preparation Process:
[0041] Add the prescribed amount of mezlocillin sodium and the prescribed amount of metronidazole lipid microspheres (calculated as metronidazole) directly to the water for injection (theoretical loading is 2ml / cartridge); stir and dissolve and then add the pH regulator Adjust the pH value of the solution so that it is in the range of 6.5-6.8; then pass through 0.45μm and 0.22μm filter membranes for sterilizing and circulating filtration for 30 minutes; after sampling and inspection meet the requirements, fill to determine the filling volume, and the second half of the filling volume is qualified Fasten the butyl rubber stopper for freeze-drying and enter the freeze-drying box for freeze-drying treatment to obtain the freeze-dried powder injection of the mezlocillin sodium c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com