Catalyst for synthesizing dimethyl carbonate through continuous oxidative carbonylation of liquid phase methanol and preparation method and application of catalyst
A technology of dimethyl carbonate and catalyst, which is applied in the field of catalyst and its preparation for the continuous oxidative carbonylation of methanol in liquid phase to synthesize dimethyl carbonate, and can solve the problems of Cl ion HCl loss, environmental pollution, large particle size of catalyst active components, etc. problems, to achieve good activity and stability, simple preparation process, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
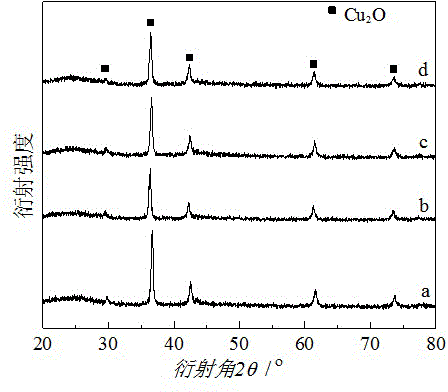
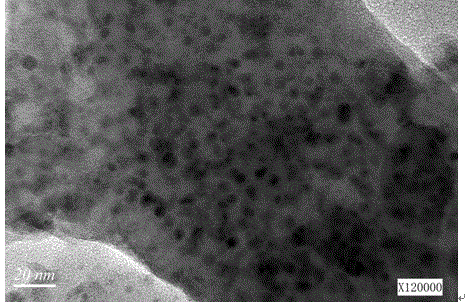
Image
Examples
Embodiment 1
[0028] (1) Weigh 49.9 g of Cu(CH 3 COO) 2 ·H 2 O, measure 500 mL of deionized water, add it to a beaker, and stir it magnetically for 10 min to form a 0.5 mol / L copper acetate solution; weigh 45.0 g of glucose C 6 h 12 o 6, measure 500 mL of deionized water, add it to a beaker, and magnetically stir for 5 minutes to form a 0.5 mol / L glucose solution; weigh 8.75 g of ammonia water, measure 500 mL of deionized water, add it to a beaker, and make a 0.5 mol / L glucose solution. L of ammonia solution.
[0029] (2) Weigh 160-200 mesh shell activated carbon (specific surface area 1600 m 2 / g, pore volume 1.2 cm 3 g -1 , micropores accounted for 12%, mesopores accounted for 80%, macropores accounted for 8%) 75.0 g, added to the above mixed solution, placed in a microwave reactor to stir the reaction, microwave temperature 70 ° C, stirring time 30 min;
[0030] (3) Wash and filter the product three times, then place it in a vacuum oven, evacuate until the vacuum degree is less t...
Embodiment 2
[0036] (1) Weigh 24.2 g of Cu(NO 3 ) 2 ·3H 2 O, measure 500 mL of deionized water, add it to a beaker, and stir it magnetically for 10 minutes to form a 0.2 mol / L copper nitrate solution; weigh 2.5 g of hydrazine hydrate, measure 500 mL of deionized water, add it to a beaker to form a 0.1 mol / L copper nitrate solution; mol / L hydrazine hydrate solution; weigh 9.0 g of urea, measure 500 mL of deionized water, add it to a beaker, and magnetically stir for 2 minutes to form a 0.3 mol / L urea solution.
[0037] (2) Weigh 240-300 mesh coconut shell activated carbon (specific surface area 2400 m 2 / g, pore volume 1.1 cm 3 g -1 , micropores accounted for 13%, mesopores accounted for 75%, macropores accounted for 12%) 90.0 g was placed in a beaker, copper nitrate solution and hydrazine hydrate solution were added in parallel, stirred, and stood for 10 minutes, then added urea solution, and then The reaction was stirred in a microwave reactor, the microwave temperature was 60°C, and...
Embodiment 3
[0041] (1) Weigh 100.0 g of CuSO 4 ·5H 2 O, measure 500 mL of deionized water, add it to a beaker, and stir magnetically for 10 min to form a 0.8 mol / L copper sulfate solution; weigh 88.2 g of sodium citrate, measure 500 mL of deionized water, add it to a beaker, and magnetically Stir for 10 minutes to form a 0.6 mol / L sodium citrate solution; weigh 20.0 g of sodium hydroxide, measure 500 mL of deionized water, add it to a beaker, and magnetically stir for 1 minute to form a 1.0 mol / L sodium hydroxide solution.
[0042] (2) Weigh 200-240 mesh shell activated carbon (specific surface area 1800 m 2 / g, pore volume 1.2 cm 3 g -1 , micropores accounted for 18%, mesopores accounted for 65%, macropores accounted for 17%) 200.0 g was placed in a beaker, copper sulfate solution and sodium citrate solution were added in parallel, stirred, and stood for 10 minutes, then added sodium hydroxide solution, and then placed in a microwave reactor to stir the reaction, the microwave temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com