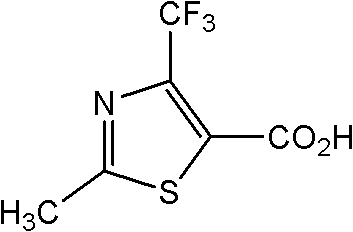
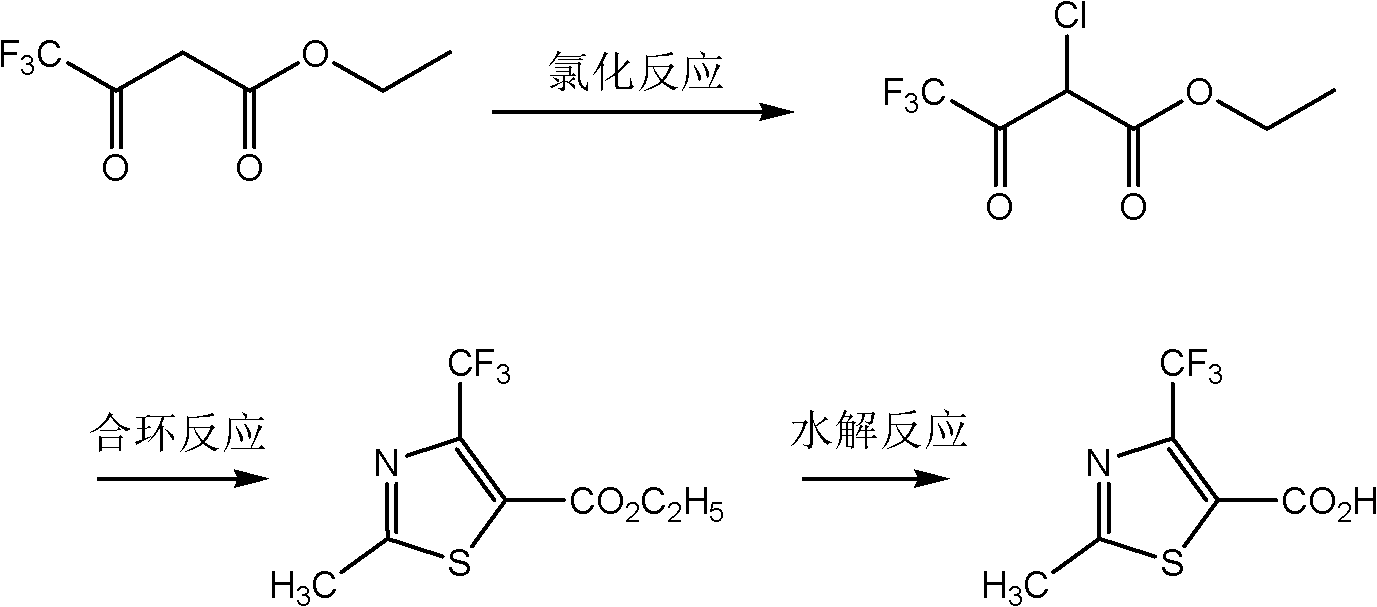
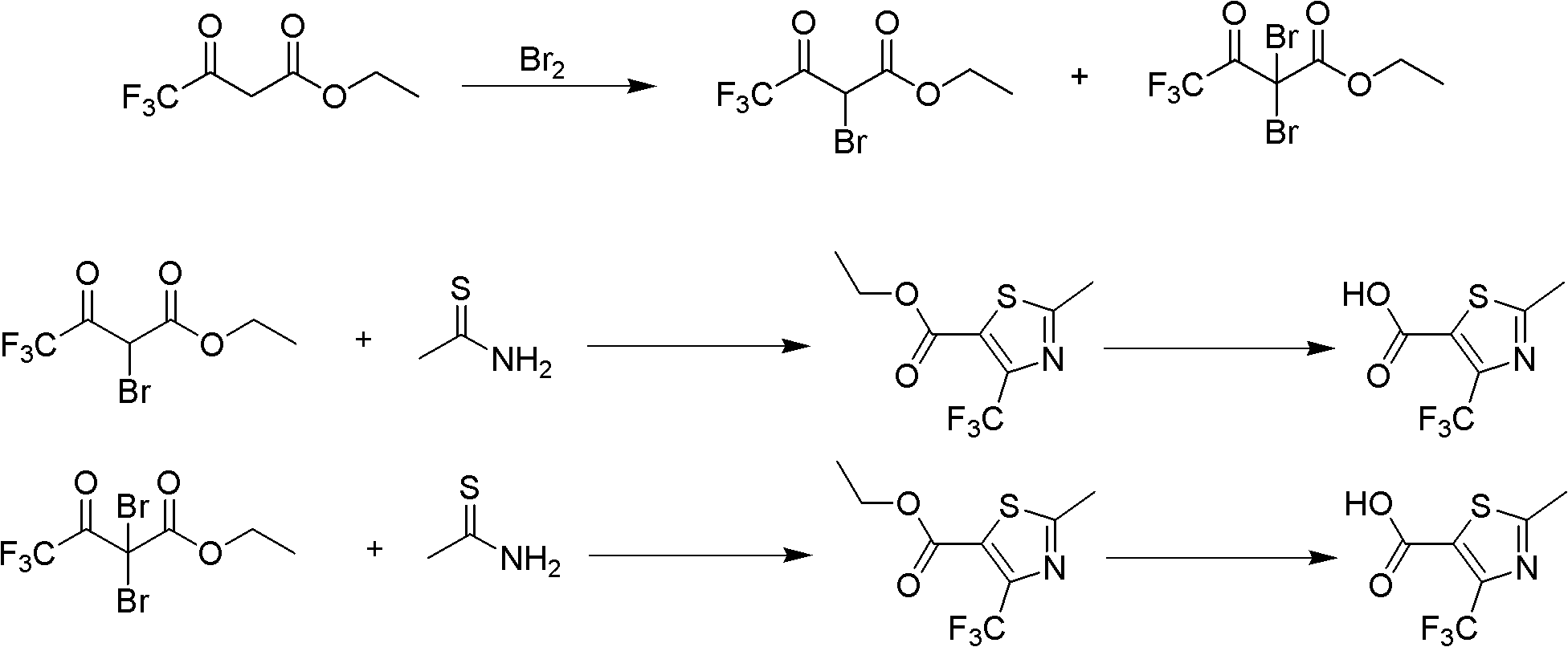
Preparation method for 2-methyl-4-(trifluoromethyl)thiazole-5-formyl acid
A technology of trifluoromethyl, bromotrifluoroethyl acetoacetate is applied in the field of preparation of 2-methyl-4-thiazole-5-carboxylic acid, and can solve the problem of low utilization rate of ethyl trifluoroacetoacetate and lower reaction temperature , low reaction yield and other problems, to achieve the effects of convenient and easy-to-obtain raw materials, simple process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of ethyl 2-bromotrifluoroacetoacetate and ethyl 2,2-dibromotrifluoroacetoacetate
[0032] The reactor is a 1000ml three-necked flask equipped with a stirring device, a thermometer and a dropping funnel. After adding 192 g of liquid bromine (1.2 mol), 184 g of ethyl trifluoroacetoacetate (1.0 mol) and 300 ml of dichloromethane were added to the reactor at room temperature. After stirring and reacting at room temperature for 10 hours, the reaction solution was washed with saturated sodium bicarbonate solution and saturated salt until neutral, and 260 g of ethyl 2-bromotrifluoroacetoacetate and 2,2-dibromotrifluoroacetoacetate were obtained after precipitation. A mixture of ethyl esters containing 95.3% ethyl 2-bromotrifluoroacetoacetate and 4.3% ethyl 2,2-dibromotrifluoroacetoacetate. The yield of the bromination reaction was 97.48%. This mixture is directly used as starting material for the cyclization reaction.
Embodiment 2
[0033] Embodiment 2: Preparation of ethyl 2-methyl-4-(trifluoromethyl)thiazole-5-carboxylate
[0034]Add 30 g (0.11 mol) of a mixture of ethyl 2-bromotrifluoroacetoacetate and ethyl 2,2-dibromotrifluoroacetoacetate into a reactor with a stirring device, and start adding sulfur dropwise after cooling to 0°C Acetonitrile solution of thioacetamide, which contains 11.3 g (0.15 mol) of thioacetamide and 50 ml of acetonitrile. During the dropwise addition of the acetonitrile solution of thioacetamide, the exothermic heat of the system increases the reaction temperature, and the rate of addition is controlled so that the temperature in the system does not exceed 5°C. Yellow solids are continuously precipitated during the stirring reaction. The dropping time is 150min. After the acetonitrile solution of thioacetamide has been added dropwise, it is warmed up to room temperature, and at room temperature, 30.3 g (0.3 mol) of triethylamine is started to be added dropwise, and the time fo...
Embodiment 3
[0035] Embodiment 3: Preparation of 2-methyl-4-(trifluoromethyl)thiazole-5-carboxylic acid
[0036] Get the ethyl acetate solution of the 2-methyl-4-(trifluoromethyl)thiazole-5-formic acid ethyl ester (0.102mol) of 115g, add dropwise in the reactor 50g content and be 40% Hydroxide Sodium solution, control the rate of addition so that the internal temperature of the system is lower than 40 ° C, after the sodium hydroxide solution is added dropwise, keep the reaction for 60 minutes. After the heat preservation is over, separate the liquid, take the organic phase, slowly add 10% hydrochloric acid to it, and adjust the pH of the system to ≤ 2. At this time, there is a large amount of precipitation, filter the product, and wash the filter cake twice with 10% hydrochloric acid Then put it in a vacuum oven for drying. Obtain light yellow 2-methyl-4-(trifluoromethyl)thiazole-5-formic acid powdery solid 21.5g, wherein the content of 2-methyl-4-(trifluoromethyl)thiazole-5-formic acid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com