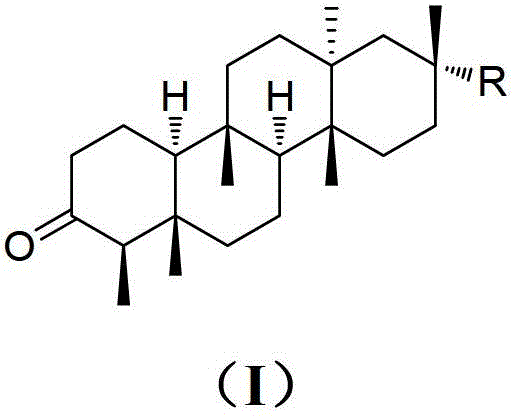
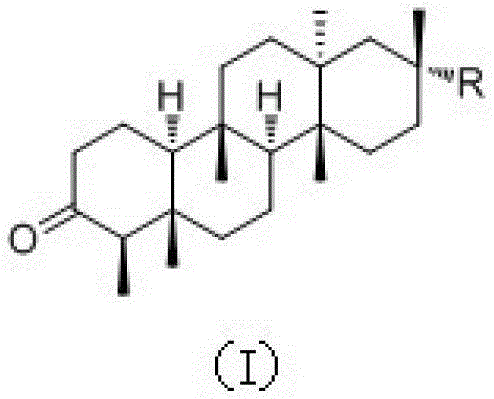
Shionone triterpenes as well as pharmaceutical compositions, preparation methods and applications of shionone triterpenes
A kind of technology of asterone and aster, applied in hepatitis B virus inhibitor, application in health care products and food, preparation of anti-hepatitis B virus medicine field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
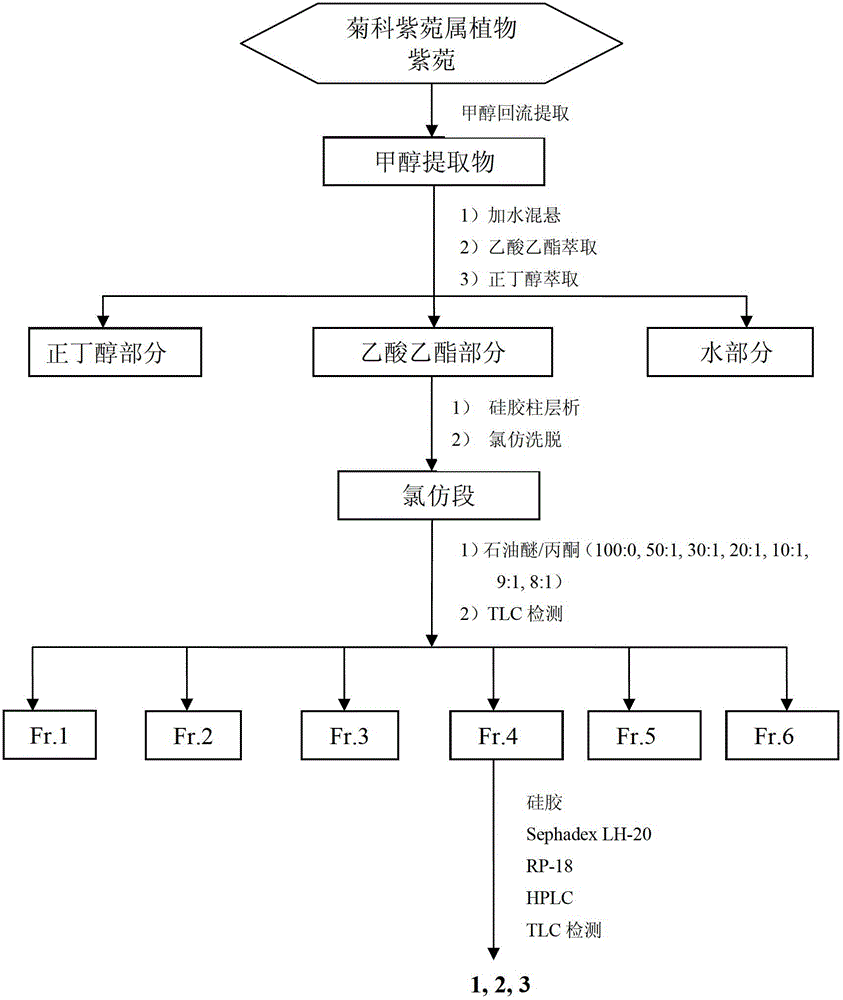
[0033] Preparation and structural identification of new shionone-type triterpenes astatarone C(1), astatarone G(2) and astatarone H(3) from Aster genus:
[0034] Take 50kg of roots and rhizomes of Aster genus Aster, after drying and crushing, soak in 90% industrial methanol for 5 hours, then heat to 65°C for reflux extraction for 3 times (200L×3 times), the first time is 3 hours, For the second time and the third time respectively for 2 hours, the extract was concentrated under reduced pressure to obtain 23kg of methanol extract; ×3 times), the solvent was recovered to obtain 2kg of ethyl acetate, 5L of n-butanol and water; the ethyl acetate was subjected to silica gel (100-200 mesh) column chromatography, and eluted with chloroform to obtain a chloroform segment; The chloroform section was subjected to silica gel (100-200 mesh) column chromatography, eluted with petroleum ether / acetone (100:0-8:1), combined with TLC detection method, and combined into 6 according to the polar...
Embodiment 2
[0047] Experiments on the inhibitory effect of astatarone C (1), astatarone G (2) and astatarone H (3) of the present invention on the secretion of HBsAg and HBeAg from HepG2.2.15 cells and their toxicity to HepG2.2.15 cells. The specific experimental principles, methods and results are as follows:
[0048] Anti-HBV experimental principle: HepG2.2.15 cell line is a transfected cell line established after stably transfecting the whole HBV genome. HepG2.2.15 cells can secrete complete HBV viral DNA and express HBV antigens stably for a long time. It is a good cell model for screening anti-HBV drugs and drug evaluation in vitro, and has been widely used in in vitro research on anti-HBV drugs. . The test compound was co-cultured with HepG2.2.15 cells, and the level of HBsAg and HBeAg secreted by the cells after administration was detected by the HBV antigen detection kit, which can reflect the anti-HBV effect of the compound in vitro.
[0049] Anti-HBV experimental method: (1) C...
Embodiment 3
[0058] Compound 1-3 obtained in Example 1 was added with 5% sodium hydroxide or ammonia solution, pH = 14, filtered, and dried to prepare sodium or ammonium salt compound 1-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com