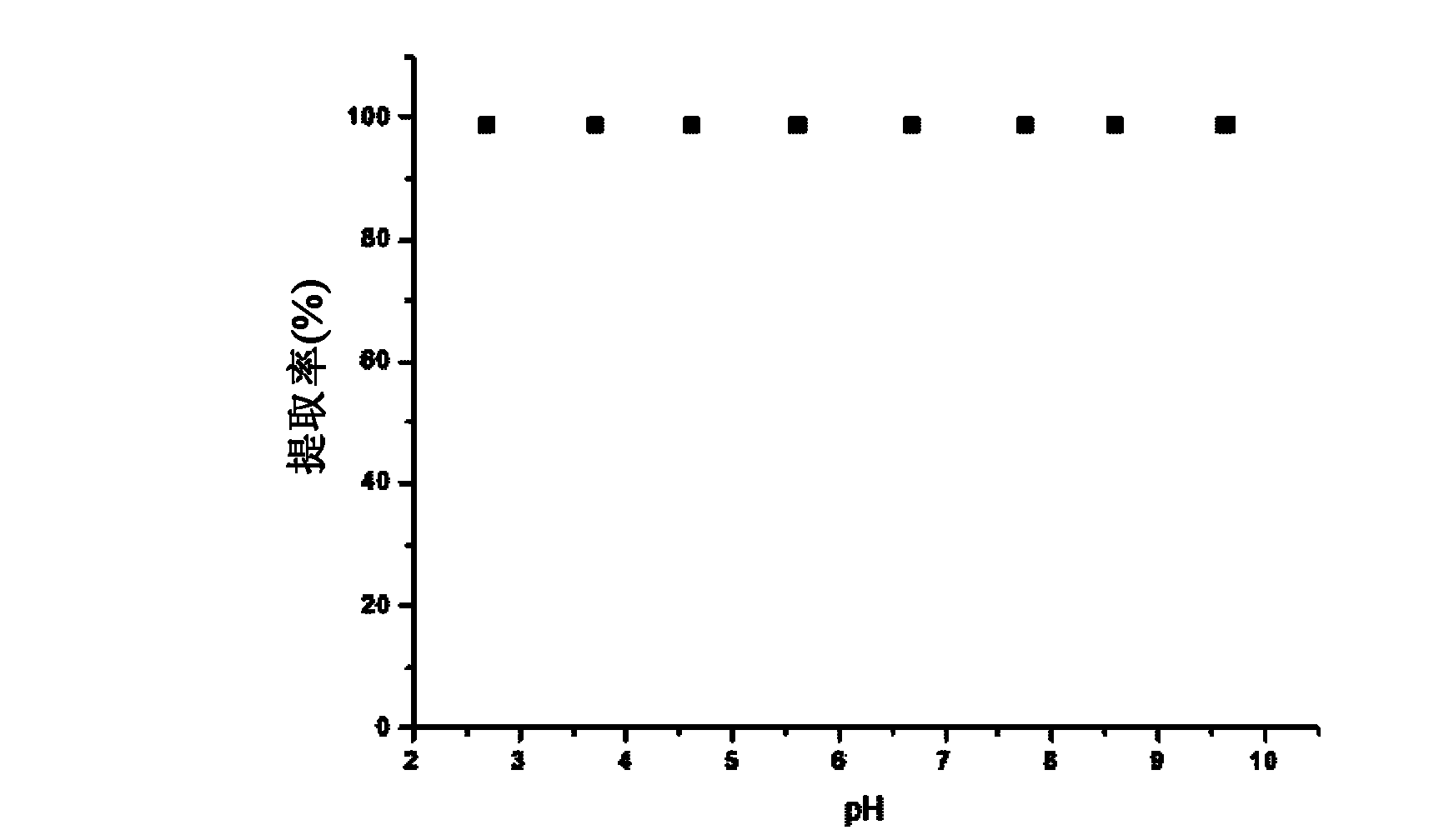
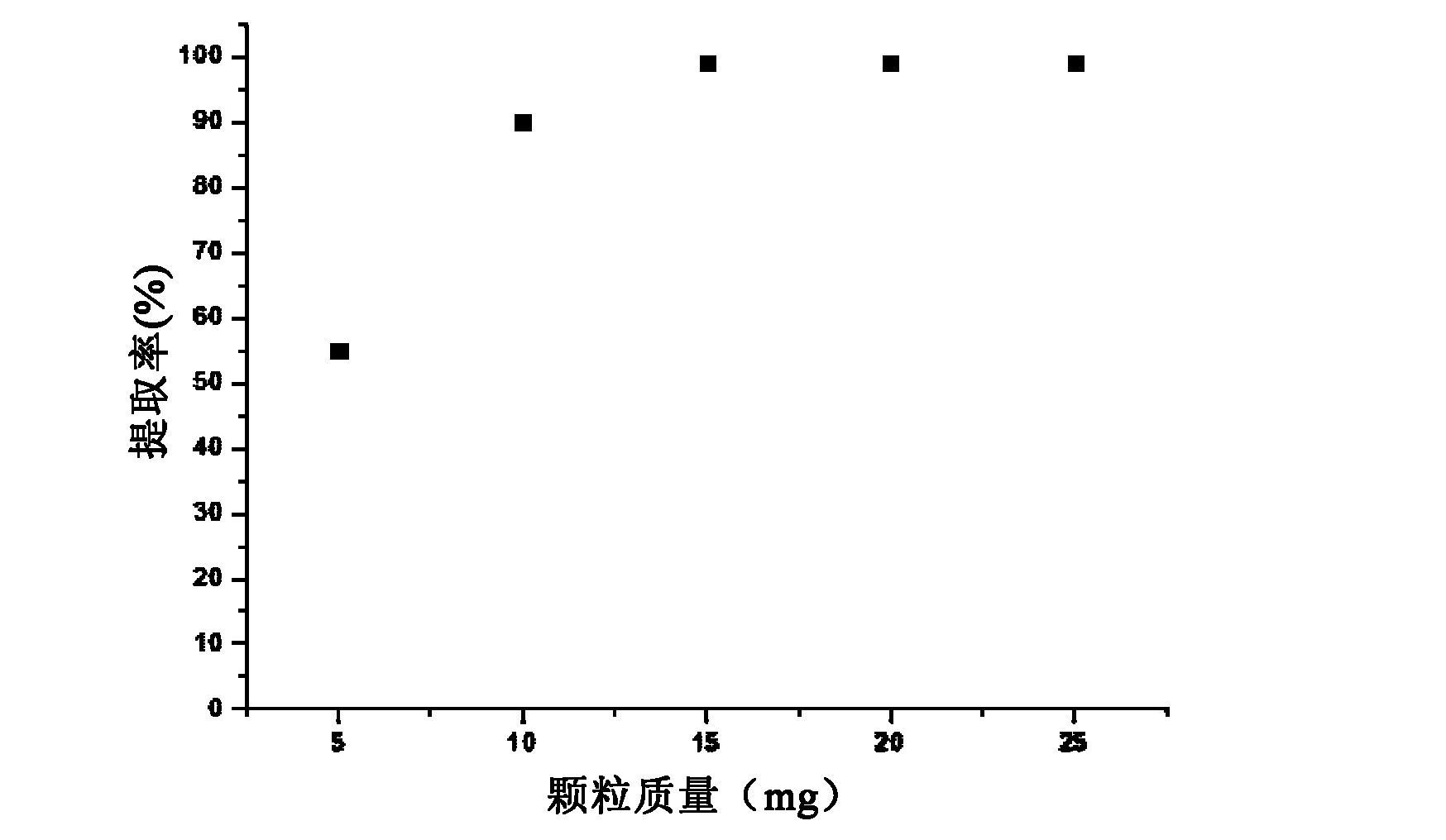
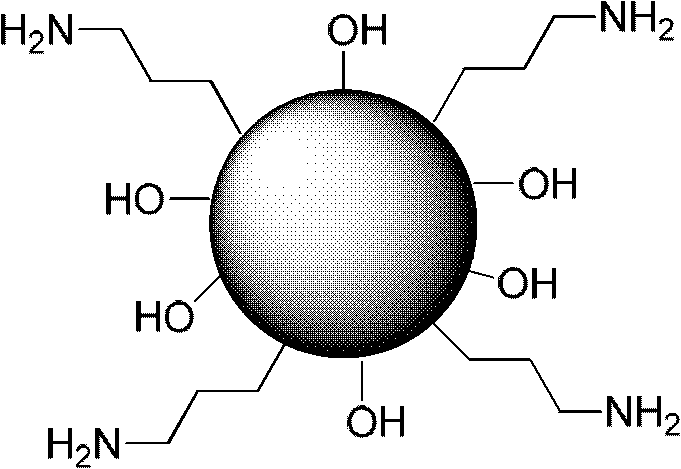
Material adsorbing heavy metal in water solution and method
A technology for heavy metal ions and separation materials, which is applied in the fields of adsorbed water/sewage treatment, solid adsorbent liquid separation, separation methods, etc., and can solve the problem of inability to remove or separate heavy metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068]
[0069] In a 250mL round bottom flask, mix 0.83g NaOH (20.75mmol) and 1.52g cetyltrimethylammonium bromide (CTAB, 4.2mmol) in 80mL water, stir at 80°C for 30min until CTAB is completely dissolved. in the water. Subsequently, 1.24 g of 3-(triethoxysilane) propylamine (5.6 mmol) was added, and after stirring for 2 h at 80° C., 7 mL (3.46 mmol) of ethyl orthosilicate was added dropwise, and the addition was completed in 30 min. After continuing to stir at ℃ for 2 h, stop the reaction, and filter with hot suction to obtain a white filter cake. After the filter cake was dried at 90°C, it was uniformly dispersed in methanol (200 mL) containing 10 mL of hydrochloric acid, stirred at reflux for 24 hours, filtered to obtain a white solid, and dried to obtain amino-functionalized mesoporous silica particles.
[0070]
[0071] Weigh 500 mg of ethylene glycol diethyl ether diamine tetraacetic acid (EGTA, 1.3 mmol) and mix it in 50 mL of deionized water. Then prepare 2M NaO...
Embodiment 2
[0073]
[0074] Magnetic Fe 3 o 4 The particles were dispersed in chloroform, added to 10 mL of an aqueous solution containing 1.5 g of CTAB, and stirred for 30 minutes to obtain an oil-water emulsion, then heated to 60°C and stirred for 10 minutes to volatilize the chloroform. Add the above mixture into 60 mL of water and 0.6 mL of 2M sodium hydroxide solution, stir and heat to 70°C. Then 1 mL (tetraethyl orthosilicate) TEOS was added. After 10 minutes 100 μL of 3-aminopropyltriethoxysilane (APTES) was added and stirring was continued for 3 hours. Centrifuge, wash with ethanol three times, add the particles to a hydrochloric acid ethanol solution with Ph=1.4, stir at 60° C. for 3 hours, centrifuge, wash with ethanol three times, and dry to obtain magnetic mesoporous silica gel nanoparticles with surface amino functionalization.
[0075]
[0076] Weigh 500 mg of ethylene glycol diethyl ether diamine tetraacetic acid (EGTA, 1.3 mmol) and mix it in 50 mL of deionized wa...
Embodiment 3
[0078]
[0079] Weigh 500 mg of ethylene glycol diethyl ether diamine tetraacetic acid (EGTA, 1.3 mmol) and mix it in 50 mL of deionized water. Then prepare 2M NaOH solution, adjust the pH of EGTA aqueous solution to about 5, and stir evenly, and the solution becomes clear. Add 200 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 1.0 mmol), and stir for 20 min. Then add 2.0 g of epoxy resin containing primary amino groups on the surface, and stir overnight at room temperature. After stopping the reaction, filter with suction, and wash the resin particles several times with deionized water. The resin particles were taken out and dried at 90° C. to obtain acceptor-modified epoxy resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com