Preparation method of (R)-N-bromine-methyl naltrexone and naltrexone derivatives
A technology of bromomethylnaltrexone and naltrexone, applied in the field of naltrexone derivatives, can solve problems such as unmentioned chiral orientation rate, and achieve the effects of convenient industrial production, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: Preparation of the first naltrexone derivative
[0073] This example is used to illustrate 3-[[2-(trimethylsilyl)ethoxymethyl]oxy]-4,5α-epoxy-14-hydroxyl-17-cyclopropylmethyl-6- Ketomorphinans and methods for their preparation.
[0074] (1) Dissolve 11 grams (ie 32 mmol) of naltrexone (ie 17-(cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxy-6-oxomorphinan) in 70 ml In dichloromethane, then add 5.6 grams (i.e. 34 mmol) 2-(trimethylsilyl) ethoxymethyl chloride (abbreviated as SEMCl), 100 mg of dimethylaminopyridine (abbreviated as DMAP) and 7 ml Triethylamine, stirred at room temperature until the reaction is complete;
[0075] (2) Pour the reaction solution obtained in step (1) into 100 milliliters of saturated aqueous sodium bicarbonate solution, stir for 5 minutes, let stand to separate layers, separate the upper water layer, and then use 50 milliliters of dichloromethane for the water layer to extract;
[0076] (3) Combine the dichloromethane layers in...
Embodiment 2
[0081] Embodiment 2: Preparation of the second naltrexone derivative
[0082] This example is used to illustrate the -14-hydroxy-6-ketomorphinan iodide and its preparation method.
[0083] To 10 g (ie 21.2 mmol) 3-[[2-(trimethylsilyl)ethoxymethyl]oxy]-4,5α-epoxy-14-hydroxyl-17-cyclopropylmethyl- 20 ml of methyl iodide was added to the 6-ketomorphinan, stirred evenly, and heated to 42° C. for 16 hours. Finally, excessive iodomethane was distilled off under reduced pressure (among them, iodomethane can be recycled and reused) to obtain 13 g of yellow powdery solid with a yield of 99.9%.
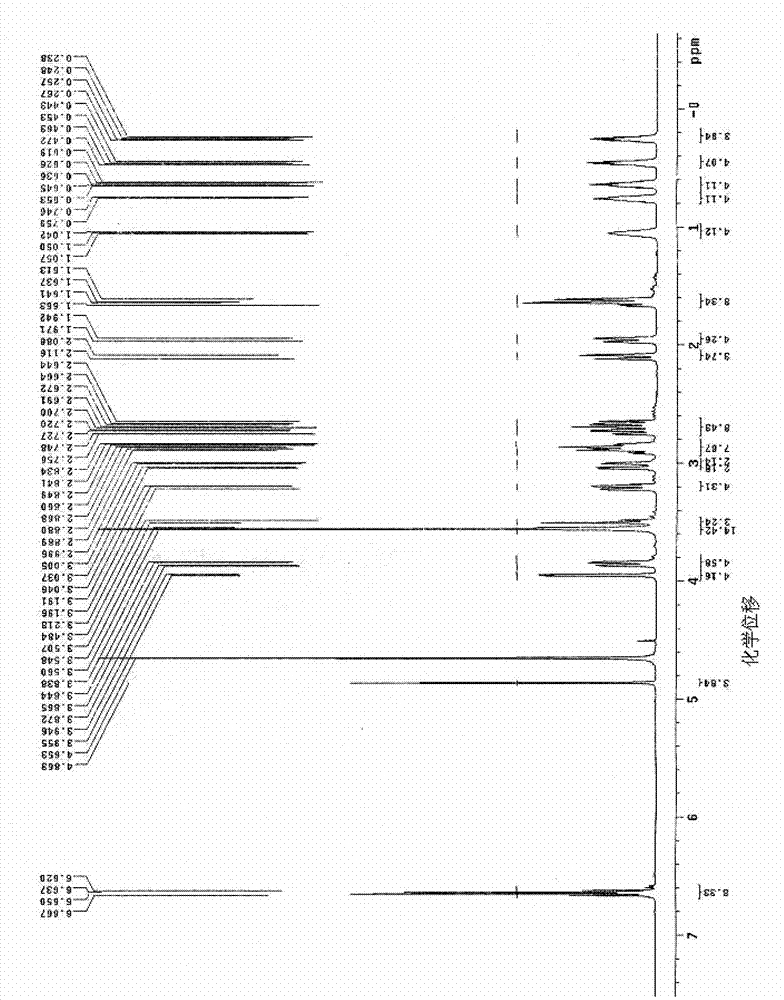
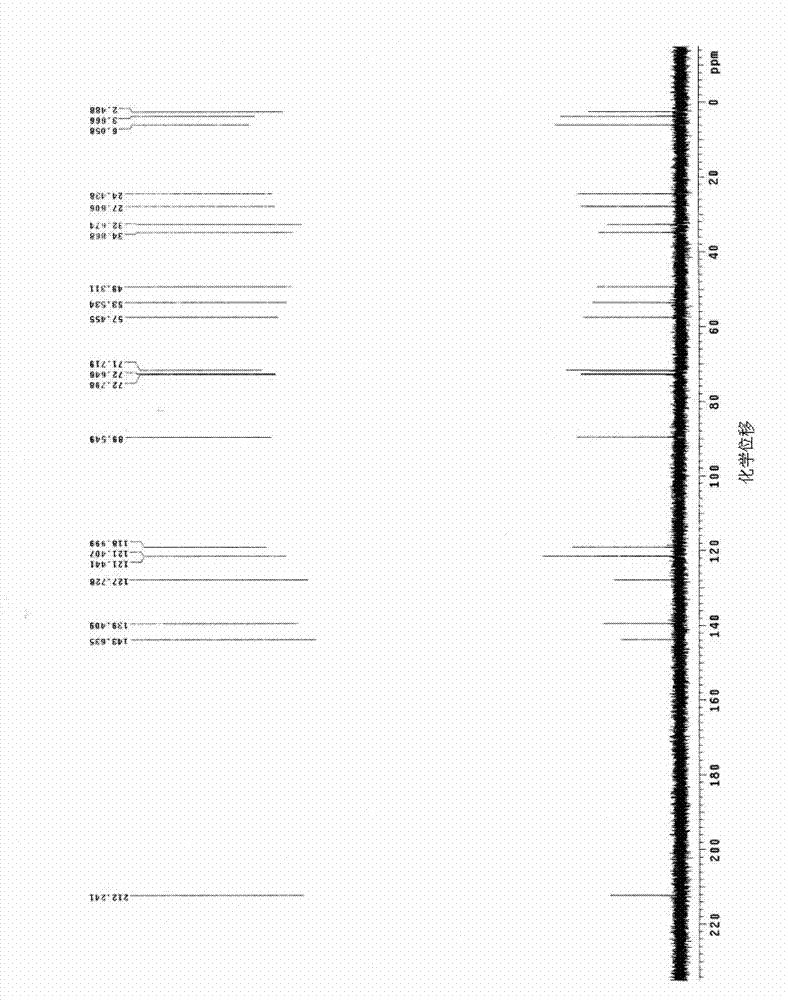
[0084] The proton nuclear magnetic resonance spectrum (abbreviation for short) of the yellow powdery solid that present embodiment makes 1 H-NMR) data are as follows: δ0.02(s,9H), 0.30(m,4H), 0.79(m,2H), 0.84(m,1H), 1.91(m,2H), 1.98(m,2H) , 2.22(m,2H), 3.20(m,2H), 3.24(t,2H), 3.30(m,3H), 3.40(t,2H), 3.75(m,1H), 4.79(s,1H), 6.02(s,2H), 6.33~6.46(q,2H), 9.04(s,1H). Among them, the above ...
Embodiment 3
[0089] This example is used to illustrate R-17,17-cyclopropylmethyl,methyl-3-hydroxy-4,5α-epoxy-14-hydroxy-6-ketomorphinan iodide / bromide and its preparation method.
[0090] To 10 g (ie 16.3 mmol) R-17,17-cyclopropylmethyl, methyl-3-[[2-(trimethylsilyl)ethoxymethyl]oxyl]-4,5α- Add 70 ml of methanol to epoxy-14-hydroxy-6-ketomorphinan iodide, stir to dissolve, and protect with nitrogen gas. At room temperature, 50 ml of 6% hydrobromic acid aqueous solution was added dropwise, and after the addition was completed, the temperature was raised to 60-70°C and the reaction was stirred for 5 hours. The reactant was concentrated under reduced pressure to obtain 6.8 g of light yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com