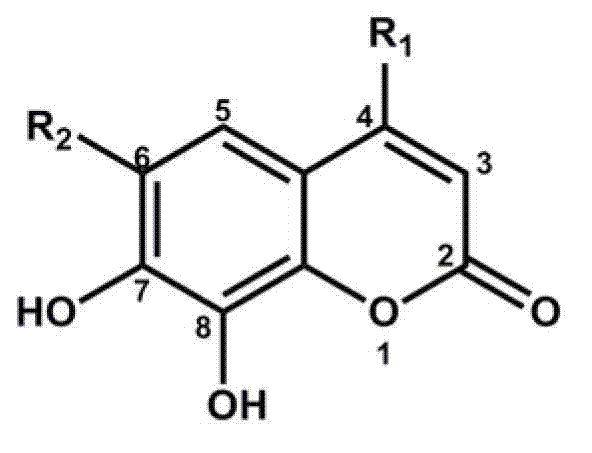
Specific probe substrate of catechol-O-methyltransgerase and application thereof
A methyltransferase and probe substrate technology, applied in the field of medicine, can solve the problems of inconvenient quantitative detection, difficult separation and use, etc., and achieve the effects of good ultraviolet absorption characteristics, easy access, and sensitive detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
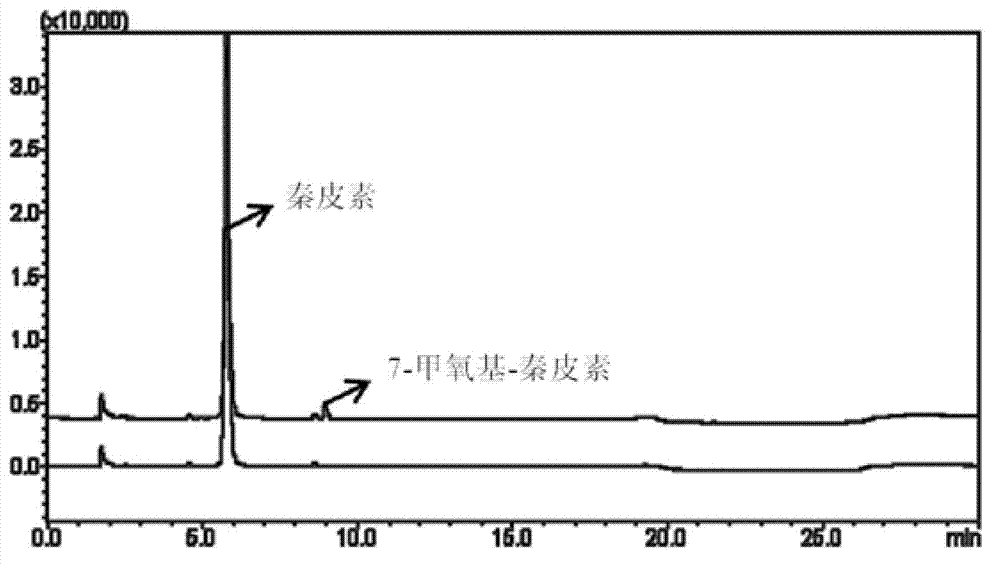
[0029] Example 1. Afracetin is used for the determination of COMT enzyme activity in 12 cases of individual human liver cytoplasm
[0030] Twelve commercialized human liver cytoplasmic samples from different individuals were purchased, and the enzyme activity of COMT in the human liver samples was determined using afracetin. The specific operation process is as follows:
[0031] (1) 40mM DTT and 5mM MgCl in 200 microliters of in vitro metabolic reaction system 2 , the concentration of human liver cytoplasm is 0.5mg / ml, the final concentration of afracetin is 100μM, pre-incubated at 37°C for 3 minutes;
[0032] (2) Add 10μl SAM (final concentration 4mM) to the reaction system to start the reaction;
[0033] (3) After 10 minutes, add 200 μl acetonitrile and shake vigorously to terminate the reaction;
[0034] (4) Use a high-speed refrigerated centrifuge to centrifuge the above system for 20 minutes under the condition of 20,000×g, and then take the supernatant for HPLC-UV dete...
Embodiment 2
[0036] Example 2. Daphnetin is used to detect enzyme activity in human brain tissue homogenate S9
[0037] Daphnetin was used to detect the COMT catalytic activity in human brain tissue homogenate S9, and the specific steps were as follows:
[0038] (1) 40mM DTT and 5mM MgCl in 200 microliters of in vitro metabolic reaction system 2 , the concentration of S9 in human brain tissue is 0.5mg / ml, the final concentration of Daphnetin is 10μM, pre-incubated at 37°C for 10 minutes;
[0039] (2) Add 10μl SAM (final concentration 4mM) to the reaction system to start the reaction;
[0040] (3) After 10 minutes, add 200 μl acetonitrile and shake vigorously to terminate the reaction;
[0041] (4) Use a high-speed refrigerated centrifuge to centrifuge the above system for 10 minutes under the condition of 20,000×g, and then take the supernatant for HPLC-UV detection and analysis;
[0042] Quantitatively detect the generation of glucuronic acid-conjugated products by ultraviolet absorpti...
Embodiment 3
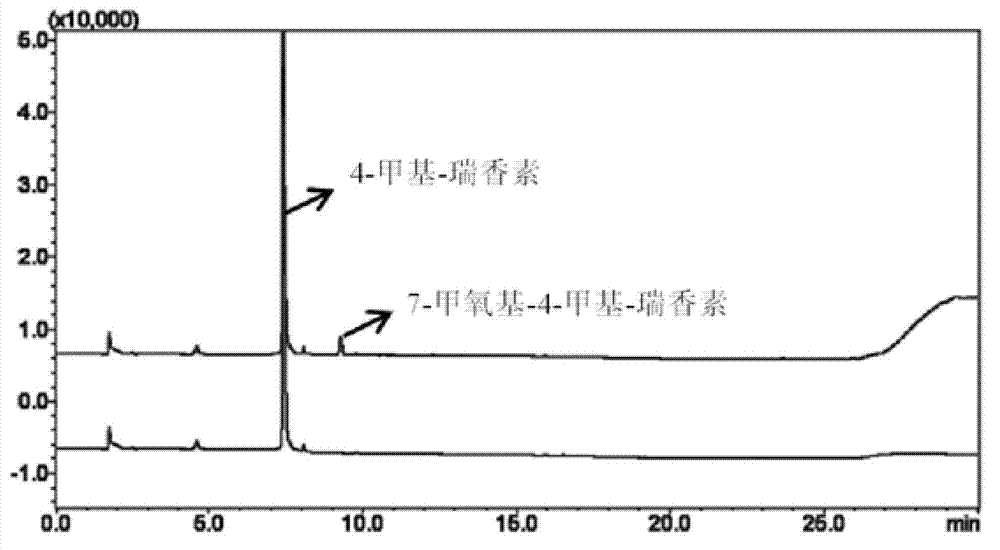
[0043] Example 3.4-Methyldaphnetin is used for the determination of COMT enzyme activity in human erythrocytes
[0044] (1) Dilute the cells with red blood cell incubation solution, place them in a 6-well culture plate, 4ml per well, put them in a metal bath shaker, 80r / min, and incubate continuously at 40°C for 120 minutes;
[0045] (2) Add 4-methyl daphnetin to the culture plate with a final concentration of 50 μM;
[0046] (3) After 30 minutes, draw 200 μl of the incubation solution and place it in a -80°C ultra-low temperature refrigerator to stop the reaction;
[0047] (4) Add 200 μl of methanol to the sample to precipitate protein, and use a high-speed refrigerated centrifuge to centrifuge the above system at 20,000×g for 20 minutes, then take the supernatant for HPLC-UV detection and analysis.
[0048] HPLC-UV was used to detect fencetin and its metabolites respectively. The results showed that the sensitivity of HPLC-UV to detect fencetin and its metabolites could rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com