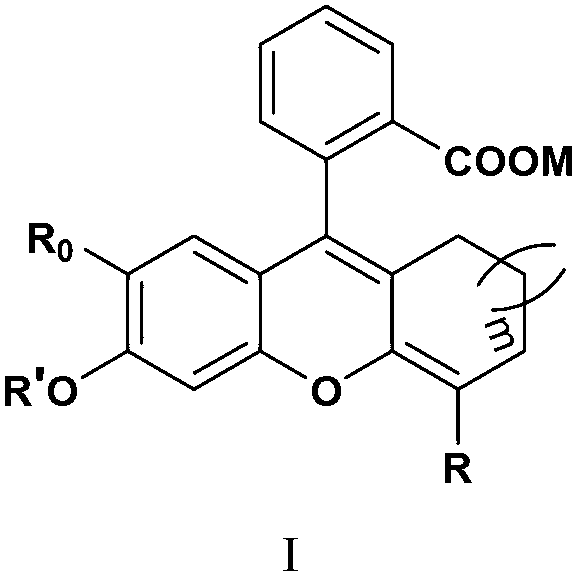
Fluorescence dye using fluorescein as matrix, and preparation method and application thereof
A fluorescent dye, fluorescein technology, applied in the field of new fluorescent dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Preparation of Fluorescent Dye Compound A 1
[0163]
[0164] (1) Synthesis of intermediate compound 1
[0165] DCF (6.30 g, 15.75 mmol) was dissolved in a round bottom flask containing 60 mL of 50% aqueous sodium hydroxide. This mixture was stirred, heated to 165°C, and stirred for 1 h, the mixture became viscous. Then the mixture was added to 400mL of ice water, neutralized with 100mL of concentrated hydrochloric acid until the acidic system became cloudy, and after standing for 2 hours, it was then filtered with a Buchner funnel, and the precipitate was washed with water for 3 times. After vacuuming, it was dried under an infrared lamp to obtain a red intermediate compound 1 as a solid (8.10 g).
[0166] (2) Synthesis of intermediate compound 2
[0167]Freshly distilled cyclohexanone (6.6 mL, 63.7 mmol) was added dropwise to concentrated sulfuric acid (7.0 mL), then cooled to 0°C. Then, intermediate compound 1 (32mmol) was added to the system in batches, the ...
Embodiment 2
[0171] Preparation of Fluorescent Dye Compound A 2 :
[0172]
[0173] (1) Dye Compound A 1 Synthesis
[0174] Prepare dye compound A according to the method of embodiment 1 1 . Obtained orange solid A 1 (1.95 g), used as starting material for the next step without further purification.
[0175] (2) Dye Compound A 2 Synthesis
[0176] Under nitrogen atmosphere, the A 1 (100mg, 0.41mmol) and malononitrile (ie vi) (40mg, 0.61mmol) were dissolved in acetonitrile (45mL, and this mixture was refluxed under nitrogen at 85°C for 4h. The reaction mixture was cooled to room temperature. Then in vacuum Solvent was distilled off under the conditions, and a dark purple solid product A was obtained on silica gel (dichloromethane as eluent: ethyl acetate=40 / 3, v / v) after purification by column chromatography 2 (0.0793g, 0.185mmol, yield 45%), R f =0.40, (5% CH 3 OH / dichloromethane). 1 H NMR (400MHz,MeOD)δ8.03(d,J=6.9Hz,1H),8.00(d,J=11.4Hz,1H),7.67–7.42(m,2H),7.10(dd,J=7.3, ...
Embodiment 3
[0178] Preparation of Fluorescent Dye Compound A 3 :
[0179]
[0180] (1) Dye Compound A 1 Synthesis
[0181] Prepare dye compound A according to the method of embodiment 1 1 . Obtained orange solid A 1 (1.95 g), used as starting material for the next step without further purification.
[0182] (2) Dye Compound A 3 Synthesis
[0183] Under nitrogen atmosphere, the A 1 (766mg, 2.0mmol) and 2-cyanoacetic acid (vii) (813mg, 7.2mmol) were dissolved in ethanol (45mL), and then 0.2mL piperidine (3.2mmol) and 0.4mL acetic acid solution were added to the reaction system, This mixture was refluxed for 10 h at 85 °C under nitrogen. The reaction mixture was cooled to room temperature. Then the solvent was distilled off under vacuum, and after purification by column chromatography, a deep red solid A was obtained on silica gel (dichloromethane / methanol=200 / 3, v / v as eluent). 3 (150mg, 0.33mmol, yield rate is 20%), Rf=0.30, (5% CH 3 OH / dichloromethane). 1 H NMR (400MHz,M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com