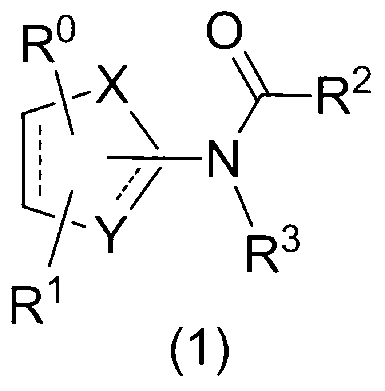
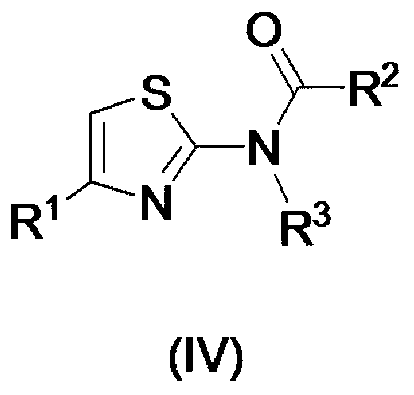
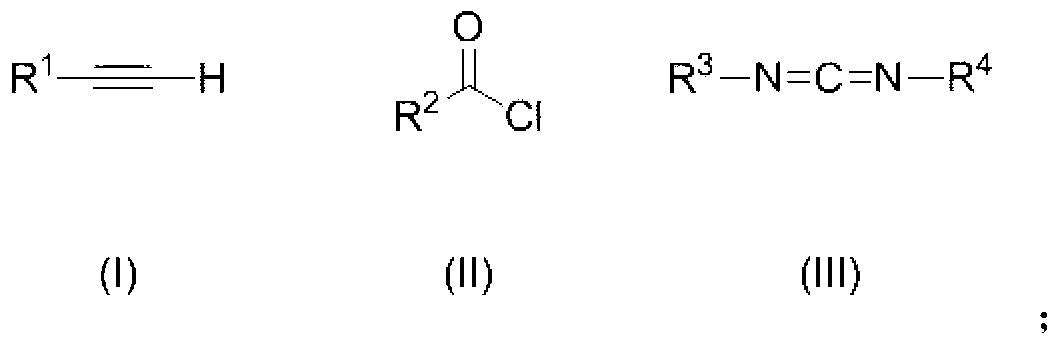
1, 3-thiazole derivative
A technology for thiazole rings and compounds, applied in the field of 2-substituted 1,3-thiazole derivatives and their synthesis, can solve problems such as cumbersome operations and restrictions on the types of substituent groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1——preparation formula IVa (R 1 = Ph, R 2 =4-CF 3 -Ph,R 3 =Et) compound:
[0091]
[0092] IVa
[0093] Under the protection of nitrogen, add 1mmol phenylacetylene to a 20mL reaction tube containing 2mL ether, use a dry ice-acetone bath to keep the reaction temperature at -78°C, add 1mmol n-butyllithium dropwise, and react for half an hour after the addition is complete. Add 0.125mmol S 8 , naturally warming up to 25°C, and reacting with magnetic stirring for two hours. Under nitrogen protection, 1 mmol of N-tert-butyl-N-ethylcarbodiimide and 1 mmol of p-trifluoromethylbenzoyl chloride were added to a 20 mL reaction tube filled with 3 mL of diethyl ether, and reacted at 25° C. for 48 h. The above two reaction solutions were mixed and reacted at 80° C. for 10 hours. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether:dichloromethane=1:1 was used as an eluent to obtain a thia...
Embodiment 2
[0094] Embodiment 2——preparation formula IVb (R 1 =2-Me-Ph, R 2 =4-CF 3 -Ph, R 3 =Et) compound:
[0095]
[0096] IVb
[0097] Under the protection of nitrogen, add 1mmol of o-methylphenylacetylene to a 20mL reaction tube containing 2mL of ether, use a dry ice-acetone bath to keep the reaction temperature at -78°C, add 1mmol of n-butyllithium dropwise, and react after the addition is complete. Half an hour, add 0.125mmol S 8 , naturally warming up to 25°C, and reacting with magnetic stirring for two hours. Under nitrogen protection, 1 mmol of N-tert-butyl-N-ethylcarbodiimide and 1 mmol of p-trifluoromethylbenzoyl chloride were added to a 20 mL reaction tube filled with 3 mL of diethyl ether, and reacted at 25° C. for 48 h. The above two reaction solutions were mixed and reacted at 60° C. for 10 hours. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether:dichloromethane=1:1 was used as an elue...
Embodiment 3
[0098] Embodiment 3——preparation formula IVc (R 1 =3-Cl-Ph,R 2 =4-CF 3 -Ph,R 3 =Et) compound:
[0099]
[0100] IVc
[0101] Under nitrogen protection, add 1mmol m-chlorophenylacetylene to a 20mL reaction tube containing 2mL ether, use a dry ice-acetone bath to keep the reaction temperature at -78°C, add 1mmol n-butyllithium dropwise, and react halfway after the addition is complete. hour, add 0.125mmol S 8 , naturally warming up to 25°C, and reacting with magnetic stirring for two hours. Under nitrogen protection, 1 mmol of N-tert-butyl-N-ethylcarbodiimide and 1 mmol of p-trifluoromethylbenzoyl chloride were added to a 20 mL reaction tube filled with 3 mL of diethyl ether, and reacted at 25° C. for 48 h. The above two reaction solutions were mixed and reacted at 100° C. for 10 hours. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether:dichloromethane=1:1 was used as an eluent to obtain a th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com