Method for removing gas-phase potassium ions in biomass flue gas by utilizing ammonium phosphate
A technology of ammonium phosphate salt and biomass, applied in separation methods, chemical instruments and methods, and separation of dispersed particles, can solve the problems of excessive ammonium sulfate spraying, and achieve the effect of avoiding ash accumulation and corrosion, and solving ash accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
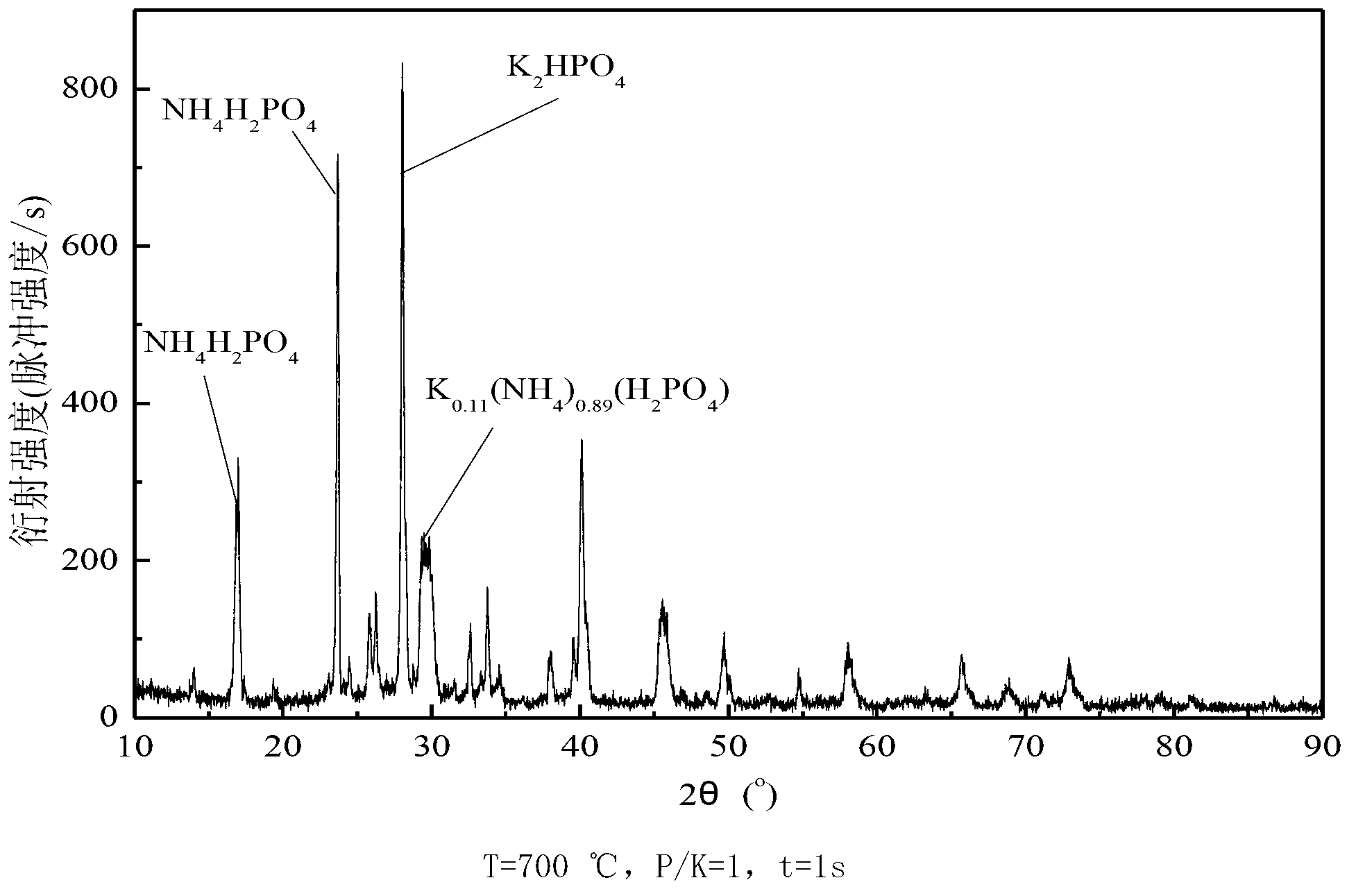
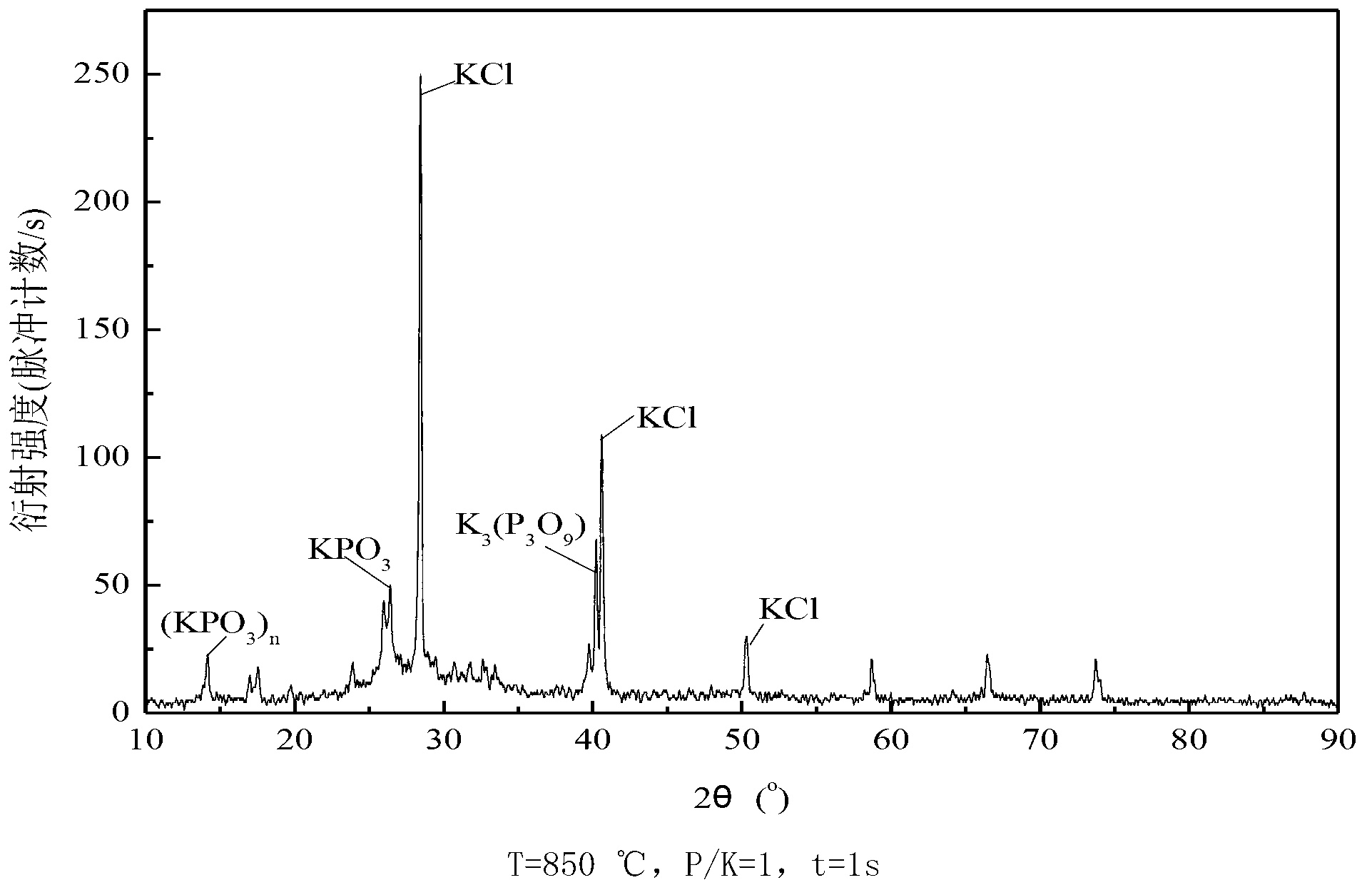
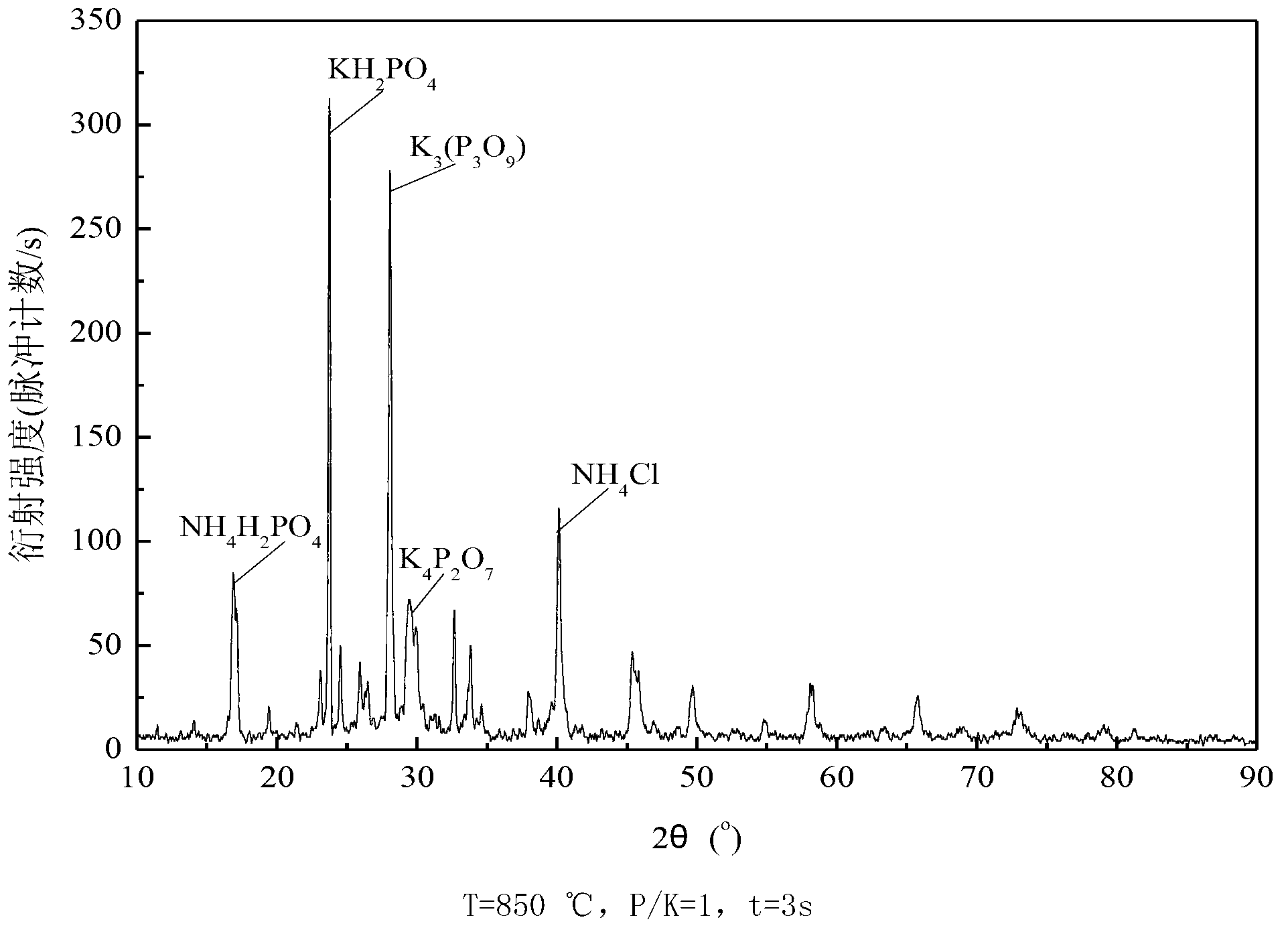
[0059] The impact of embodiment 1 residence time and phosphorus-potassium mol ratio on gas-phase potassium ion removal
[0060] Ammonium dihydrogen phosphate [NH 4 h 2 PO 4 ] as an example, weigh a certain amount of ammonium dihydrogen phosphate and potassium chloride (KCl) and mix them according to different phosphorus-potassium molar ratios.
[0061] The effect of different temperature (T), residence time (t) and phosphorus-to-potassium molar ratio (P / K) on gas-phase potassium ion removal was studied in a vertical tube furnace experimental system. The standard working condition in the experiment is: O 2 The concentration is 3%, and the experimental temperature is 700°C, 850°C, 900°C and 1000°C. The standard working conditions in the experiment are: P / K=1, the residence time is 3s respectively; at 850°C, the residence time is 1s and 3s respectively, and the molar ratio of phosphorus and potassium is 1 and 3, and its influence on the removal of potassium ions in the gas ph...
Embodiment 2
[0070] Embodiment 2 adds the [NH of the amount of different substances 4 h 2 PO 4 ] Effects on gas phase KCl removal under flue gas atmosphere
[0071] This example uses the thermodynamic simulation software FactSage to simulate the [NH 4 h 2 PO 4 ] on the effect of gas phase KCl removal in flue gas atmosphere.
[0072] The temperature selected for the simulation is 700°C, 850°C, 950°C and 1000°C respectively, and the composition atmosphere of the flue gas is O 2 3%, CO 2 16%, H 2 O 20%, N 2 56.5%, CO 0.01%, KCl 0.004%, SO 2 0.0009%, NO 0.0086%, HCl 0.0035% and other trace gases, and the amount of KCl in the gas phase is 4mol, and other gases are set in proportion. [NH 4 h 2 PO 4 ] from the initial 0 mol to 4 mol, after using the thermodynamic chemical equilibrium model Equilib in the thermodynamic simulation software FactSage to simulate, the simulation results are shown in Figure 5(a) and Figure 5(b). in,
[0073] Figure 5(a) T=700°C;
[0074] Figure 5(b)...
Embodiment 3
[0081] Embodiment 3 quantitatively [NH at different temperatures 4 h 2 PO 4 ] Effects on gas phase KCl removal under flue gas atmosphere
[0082] In KCl and [NH 4 h 2 PO 4 ] The molar ratio of substances, that is, the initial condition of P / K=0.5, simulates the reaction at different temperatures, such as Figure 6 As shown, the analysis is as follows:
[0083] As the temperature increases, the amount of KCl in the gas phase increases continuously, and the temperature rises very quickly above 950 ° C, and at the same time, the product K 2 HPO 4 The amount of the substance decreased rapidly, indicating that when the temperature exceeds 950°C, [NH 4 h 2 PO 4 ] The removal effect of gas phase KCl is not obvious.
[0084] Below 900°C, [NH 4 h 2 PO 4 ] can keep the amount of KCl in the gas phase at a low level, which indicates that [NH 4 h 2 PO 4 ] has a strong effect on the removal of KCl in the gas phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com