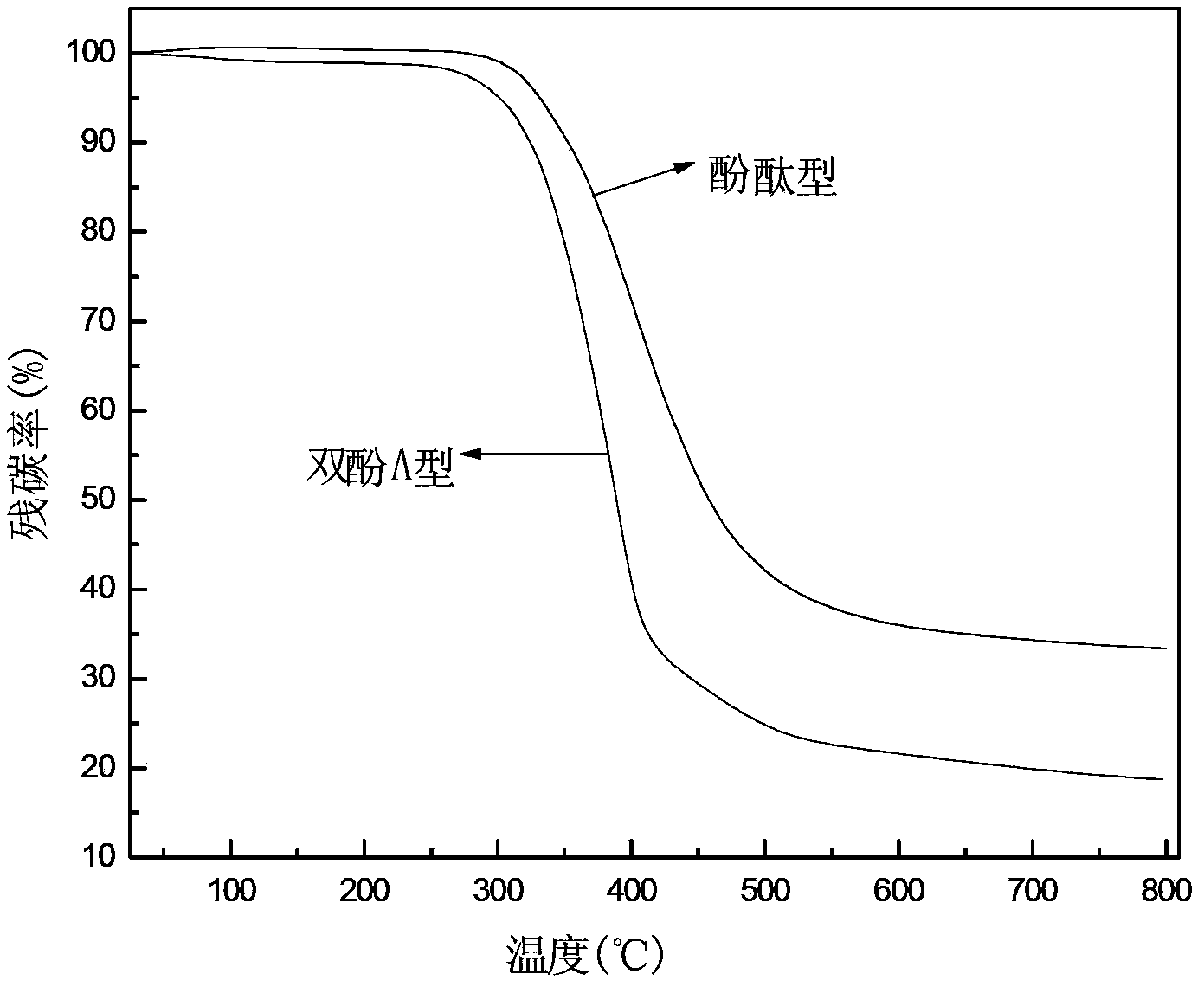
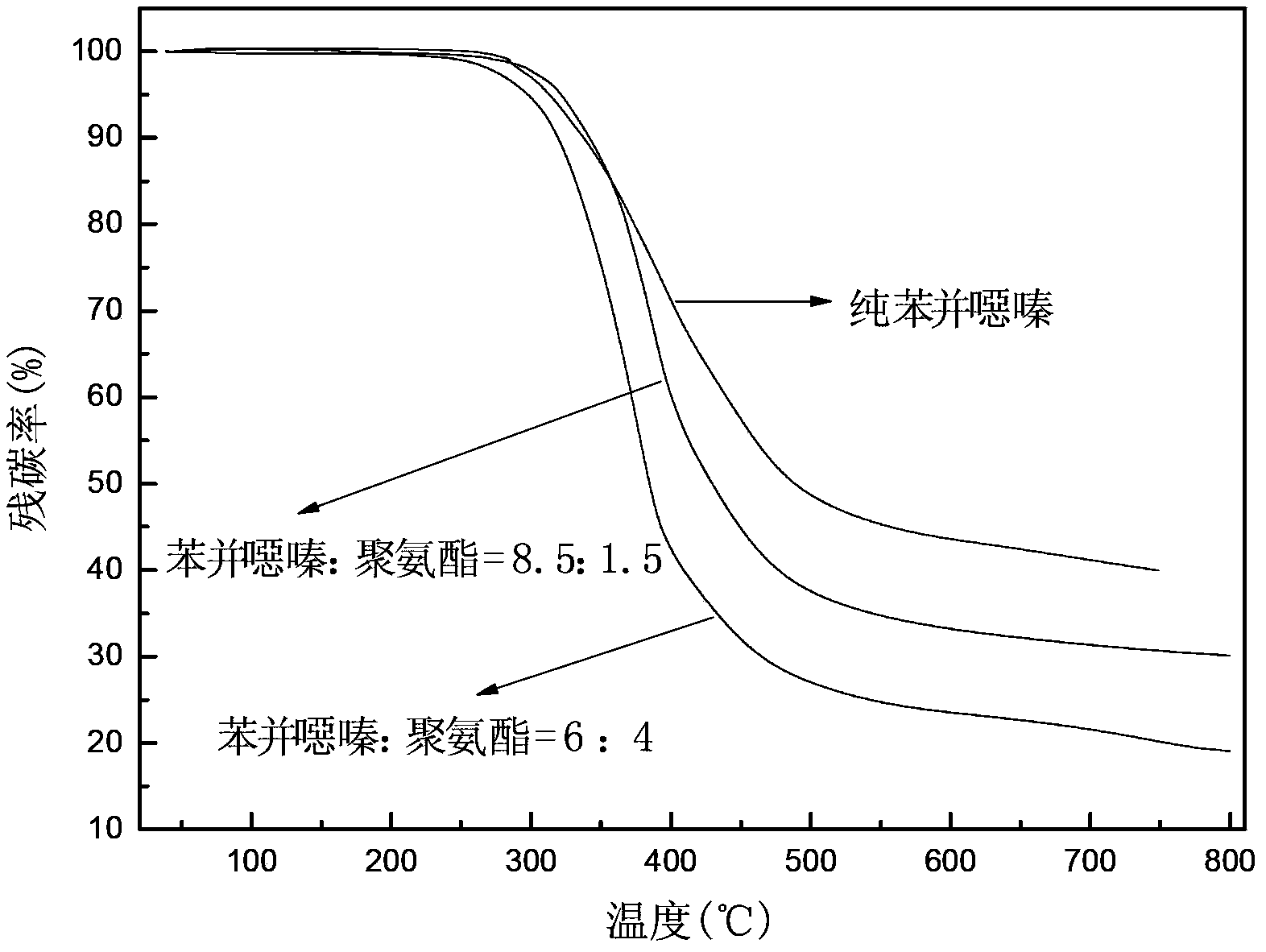
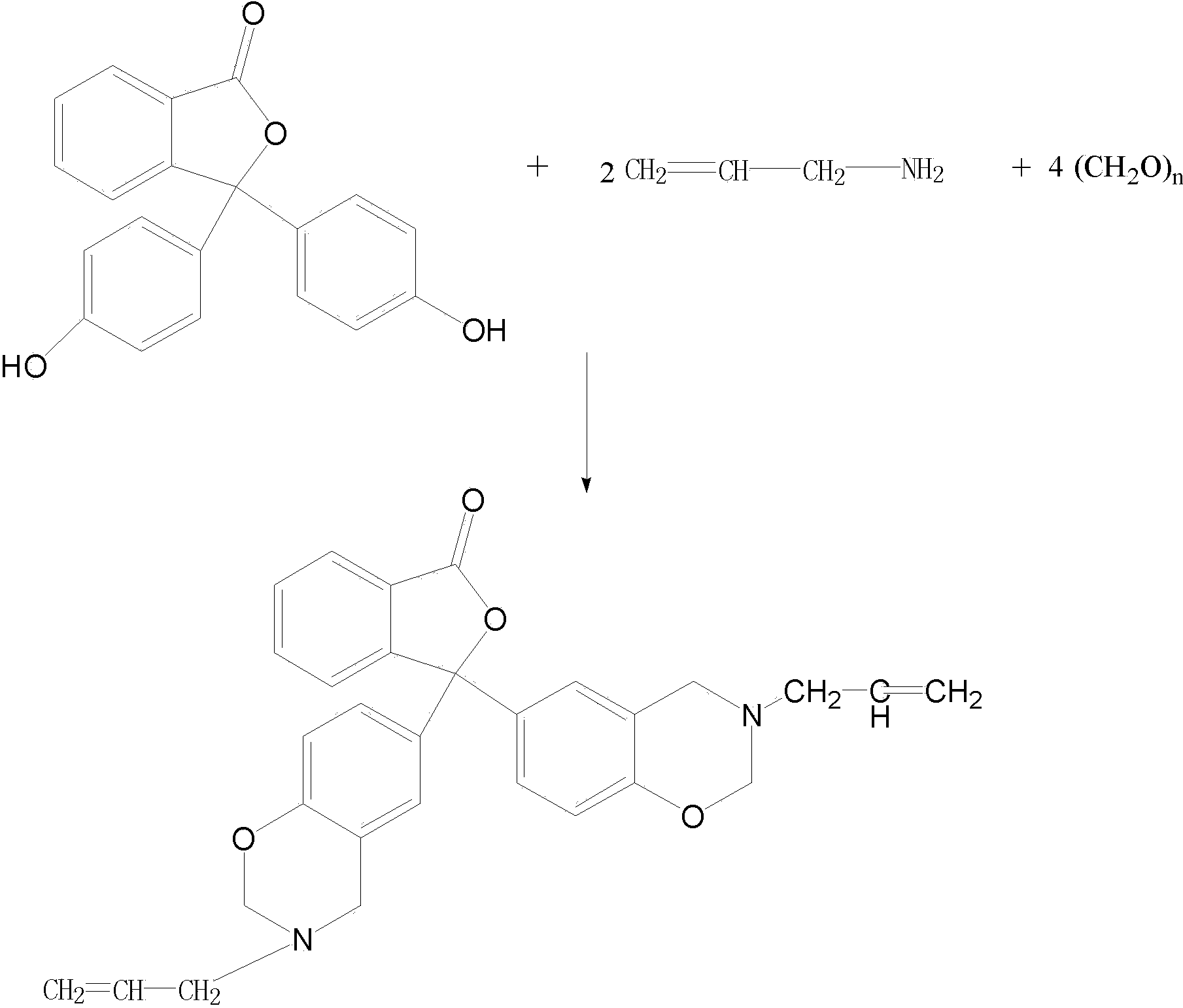
Preparation method of phenolphthalein allyl amine-type benzoxazine/polyurethane blend resin
A technology of phenolphthalein allylamine type and benzoxazine, which is applied in the field of preparation of new modified resins, can solve the problem of low decomposition temperature of mixed resin, insufficient crosslinking density of bisphenol A aniline type benzoxazine resin, bisphenol Problems such as poor thermal stability of type A resin, to achieve the effect of increasing crosslinking density, excellent flame retardancy and mechanical properties, and improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]1. Under the condition of ice bath, add 3.00g (0.1mol) paraformaldehyde and 3.75ml (0.05mol) allylamine to a 250mL three-necked flask equipped with a condenser tube and a stirring rod, and add 150ml of absolute ethanol as a solvent. After stirring for 2 hours, 7.96g (0.025mol) of phenolphthalein was added, and the temperature was raised to reflux temperature of 80°C for 8 hours to complete the reaction. The solvent was removed by rotary evaporating the reaction liquid, dried in vacuo for 3 h, the crude product after vacuum drying was dissolved in chloroform, and 2mol·L -1 The above solution is washed with NaOH, and then washed with distilled water, and then the washed solution is rotated to remove the solvent, and finally vacuum-dried to obtain the phenolphthalein allylamine-type benzoxazine.
[0034] 2. To have N 2 Add 20g (0.02mol) PPG-1000 and 50ml of solvent acetone to the protected four-necked flask, then add 8.84g (0.04mol) diisocyanate dropwise to the four-necke...
Embodiment 2
[0038] 1. Under ice bath conditions, add 6.00g (0.1mol) paraformaldehyde and 7.5ml (0.05mol) allylamine to a 250mL three-neck flask equipped with a condenser tube and a stirring rod, and add 150ml of absolute ethanol as a solvent. After stirring for 2 hours, 15.92 g (0.025 mol) of phenolphthalein was added, and the temperature was raised to reflux temperature of 80°C for 8 hours to complete the reaction. The solvent was removed by rotary evaporating the reaction liquid, dried in vacuo for 3 h, the crude product after vacuum drying was dissolved in chloroform, and 2mol·L -1 The above solution is washed with NaOH, and then washed with distilled water, and then the washed solution is rotated to remove the solvent, and finally vacuum-dried to obtain the phenolphthalein allylamine-type benzoxazine.
[0039] 2. Step 2 of this embodiment is the same as that of Embodiment 1, and a polyurethane prepolymer is prepared.
[0040] 3, the 1.8g phenolphthalein allylamine type benzoxazine p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com