Azole antifungal compound and preparation method and application thereof
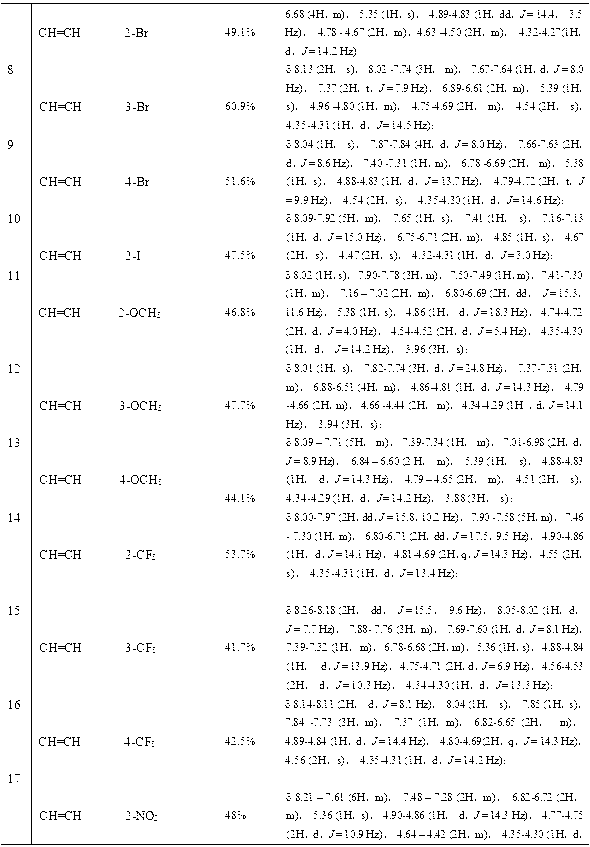
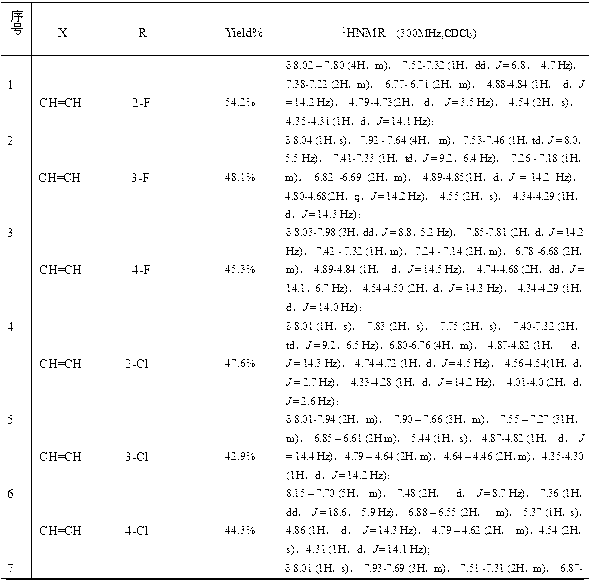
A compound, the technology of triazole alcohol, which is applied in the field of medicine and achieves the effects of simple preparation method, high yield and good antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
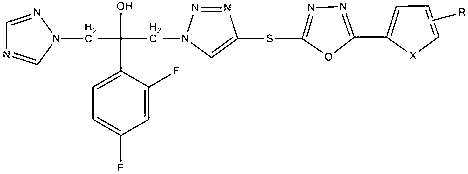
[0035] A new class of triazole alcohol compounds provided by the invention has a structure as shown in the general formula:
[0036]
[0037] Where X is CH=CH, O;
[0038] wherein R is selected from alkyl, halogen, alkoxy or nitro.
[0039] i. Alkyl, 1-5 carbon atoms of alkyl or haloalkyl, wherein the substituents can be located at the ortho, meta, or para positions of the aromatic ring, and can be mono-substituted or multi-substituted.
[0040] ii. Halogen, the substituent is selected from F, CI, Br, I, and can be located at the ortho, meta, or para position of the aromatic ring, and can be mono-substituted or multi-substituted.
[0041] iii. An alkoxy group with 1-2 carbon atoms, where the substituents can be located at the ortho, meta, or para positions of the aromatic ring, and can be mono-substituted or multi-substituted.
[0042] iv. The nitro group can be located at the ortho, meta or para position of the aromatic ring, and can be mono-substituted or multi-substitu...
Embodiment 2
[0081] The intermediate is synthesized according to the reaction scheme of a).
[0082] (1) Preparation of 2-chloro-2',4'-difluoroacetophenone
[0083] Put 200g (1.494mol) of anhydrous aluminum oxide and 150g (1.30mol) of m-difluorobenzene into a 1000mL three-necked bottle, stir at room temperature, slowly add 150g (1.30mol) of chloroacetyl chloride dropwise, and continue to Stir at room temperature for 30 minutes, slowly raise the temperature to 45°C, continue stirring at this temperature for 4.5 hours, pour the reaction solution into ice water as usual, precipitate solids, filter; the filtrate is extracted twice with 800 mL of dichloromethane, and combined Dichloromethane extract, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the solvent was recovered to obtain a solid, which was combined twice and recrystallized with ethanol to obtain 2-chloro 2',4'-difluoroacetophenone 215g, yield 88.2%, melting point: 46-47°C.
[0084] (2) Prepa...
Embodiment 3
[0091] The intermediate is synthesized according to the reaction route b), taking 4-chlorobenzoic acid as an example.
[0092] (5) Preparation of methyl 4-chlorobenzoate
[0093] Add 5g (31.9mmol) of 4-chlorobenzoic acid and 25ml of thionyl chloride to a 100ml eggplant-shaped bottle, heat to reflux for 4 hours, evaporate the solvent to dryness under reduced pressure to obtain 4-chlorobenzoyl chloride, dilute it with 15ml of dichloromethane, Add it dropwise to 20ml of methanol under ice-bath conditions. After the dropwise addition, react at room temperature for 2 hours. After the reaction, evaporate to dryness under reduced pressure to obtain 4.46g of light yellow solid methyl 4-chlorobenzoate, with a yield of 83%;
[0094] (6) Preparation of 4-chlorobenzohydrazide
[0095] Add 2.43g (14.2mmol) of methyl 4-chlorobenzoate and 25ml of methanol into a 100ml eggplant-shaped bottle, and slowly add 15ml of hydrazine hydrate solution dropwise at room temperature. solvent, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com