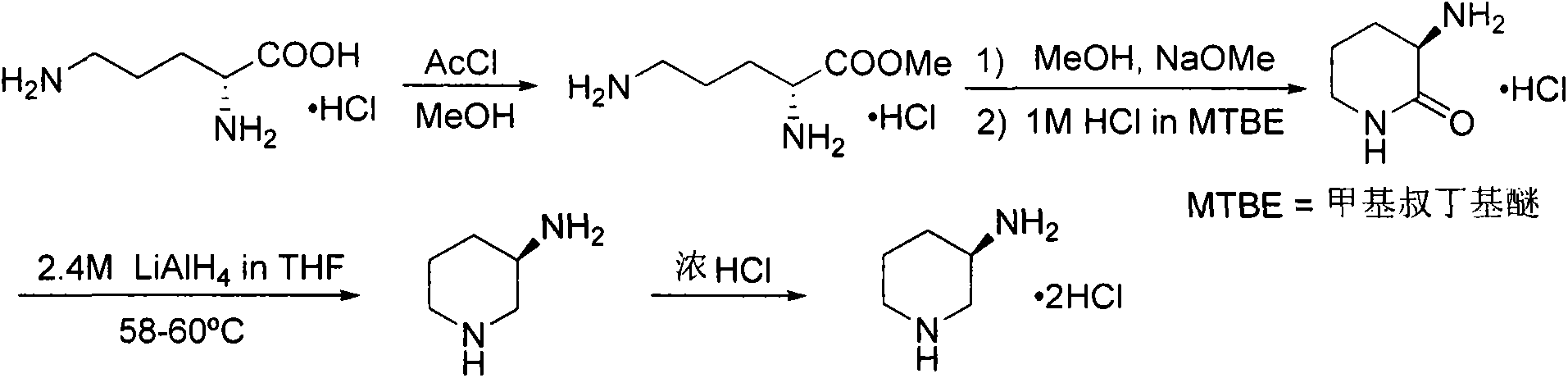
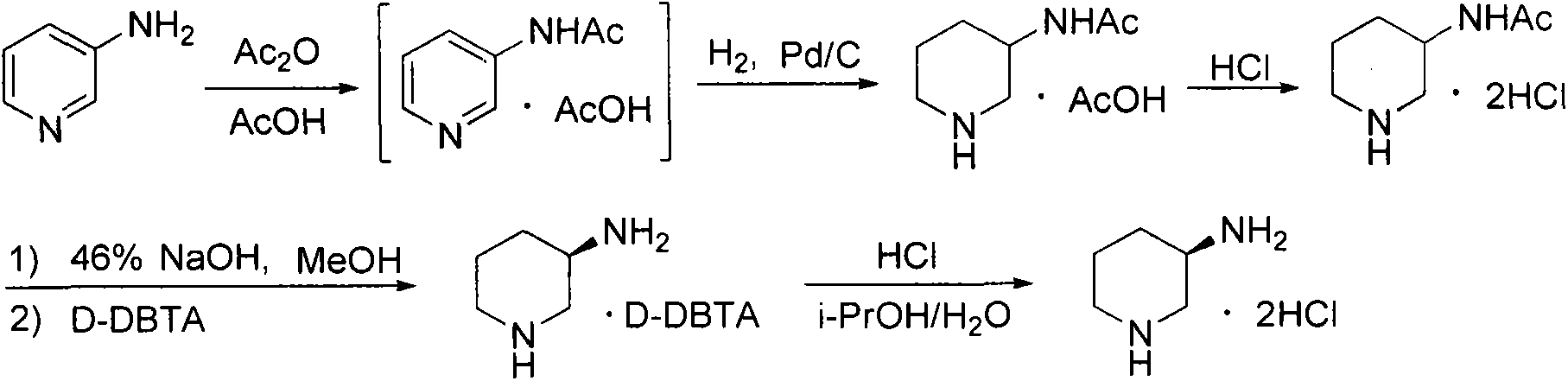
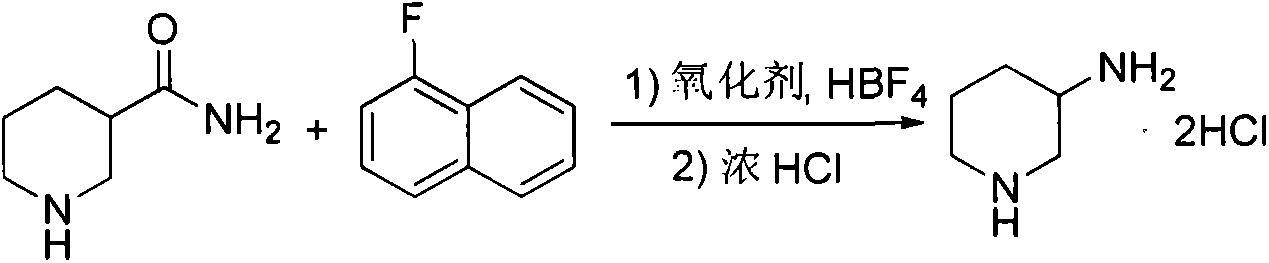
Preparation method for alogliptin intermediate R-3-aminopiperidine dihydrochloride
A technology of aminopiperidine and bishydrochloride, which is applied in the field of medicine, can solve the problems of high requirements for reaction equipment, high catalyst price, unfavorable scale-up production, etc., and achieve the effect of low price, simple reaction conditions, and easy monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 2L reaction flask, add 146g of 1-fluoronaphthalene (1mol) to a mixed solution of 925mL of ethanol and 75mL of water, add 105.4g (1.2mol) of fluoboric acid and 136g (1.2mol) of 30% hydrogen peroxide under stirring, and stir at room temperature for 0.5 After 1 hour, 128.2 g (1 mol) of 3-formamidopiperidine was added and reacted at room temperature for 8 hours. The solvent was evaporated to dryness, the residue was dispersed with 500 mL of ethyl acetate, 150 mL of concentrated hydrochloric acid was added, stirred for 0.5 hour, filtered, and dried to obtain 167.0 g of racemic 3-aminopiperidine dihydrochloride with a yield of 96.5%.
[0019] Mix 165g (0.95mol) of racemic 3-aminopiperidine dihydrochloride and 500mL of absolute ethanol in a 1L reaction flask, add sodium hydroxide under stirring to adjust the pH to neutral, then add 143g of D-tartaric acid (0.95 mol), reflux reaction for 2 hours, naturally cooled to room temperature under stirring, and filtered to obtain a...
Embodiment 2
[0022] In a 2L reaction flask, add 146g of 1-fluoronaphthalene (1mol) into a mixed solution of 900mL of ethanol and 100mL of water, add 105.4g (1.2mol) of fluoboric acid and 136g (1.2mol) of 30% hydrogen peroxide under stirring, and stir at room temperature for 0.5 After 1 hour, 128.2 g (1 mol) of 3-formamidopiperidine was added and reacted at room temperature for 8 hours. The solvent was evaporated to dryness, the residue was dispersed with 500 mL of ethyl acetate, 150 mL of concentrated hydrochloric acid was added, stirred for 0.5 hour, filtered, and dried to obtain 163.2 g of racemic 3-aminopiperidine dihydrochloride with a yield of 94.3%.
[0023] Mix 147g (0.85mol) of racemic 3-aminopiperidine dihydrochloride and 450mL of absolute ethanol in a 1L reaction flask, add sodium hydroxide under stirring to adjust the pH to neutral, then add 127.5g of D-tartaric acid ( 0.85mol), reflux reaction for 2 hours, naturally cooled to room temperature under stirring, and filtered to obt...
Embodiment 3
[0025] In a 2L reaction flask, add 146g of 1-fluoronaphthalene (1mol) to a mixed solution of 950mL of ethanol and 50mL of water, add 105.4g (1.2mol) of fluoboric acid and 136g (1.2mol) of 30% hydrogen peroxide under stirring, and stir at room temperature for 0.5 After 1 hour, 128.2 g (1 mol) of 3-formamidopiperidine was added and reacted at room temperature for 8 hours. The solvent was evaporated to dryness, the residue was dispersed with 500 mL of ethyl acetate, 150 mL of concentrated hydrochloric acid was added, stirred for 0.5 hour, filtered, and dried to obtain 164.1 g of racemic 3-aminopiperidine dihydrochloride with a yield of 94.8%.
[0026] Mix 164g (0.95mol) of racemic 3-aminopiperidine dihydrochloride and 500mL of absolute ethanol in a 1L reaction flask, add sodium hydroxide under stirring to adjust the pH to neutral, then add 142g of D-tartaric acid (0.95 mol), reflux reaction for 2 hours, naturally cooled to room temperature under stirring, and filtered to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com