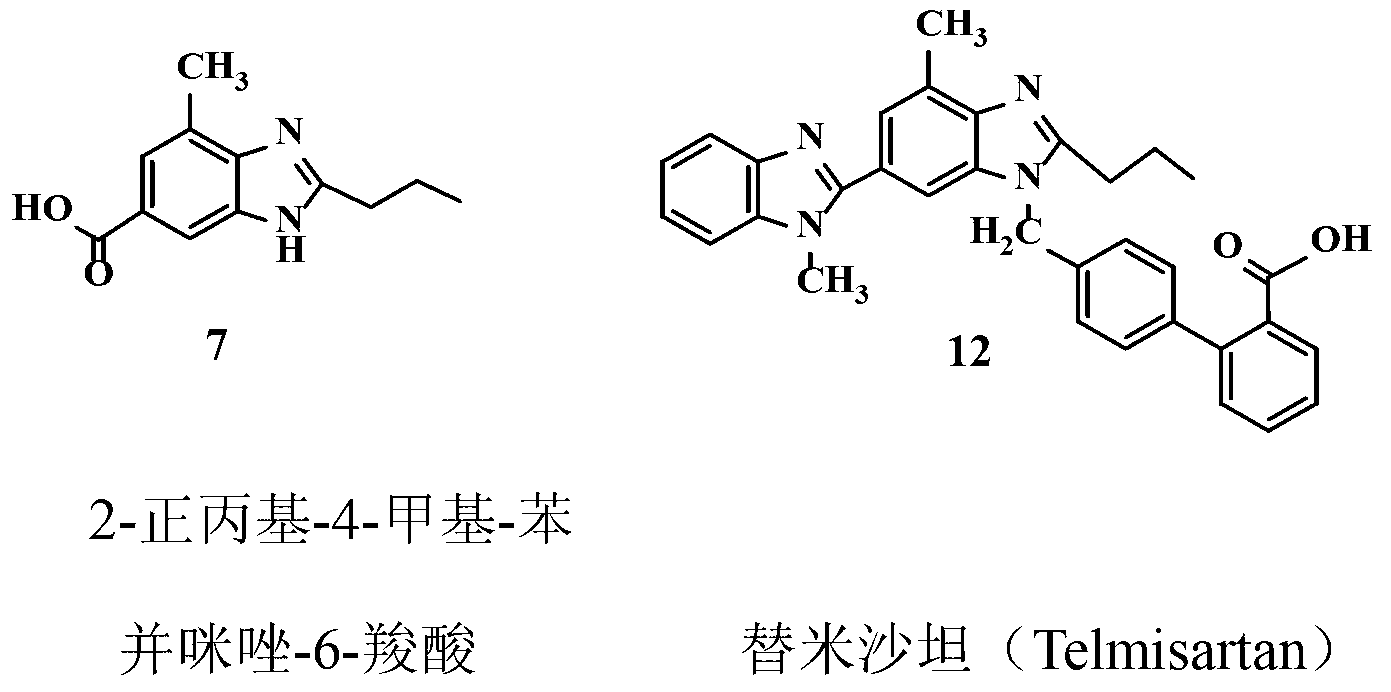
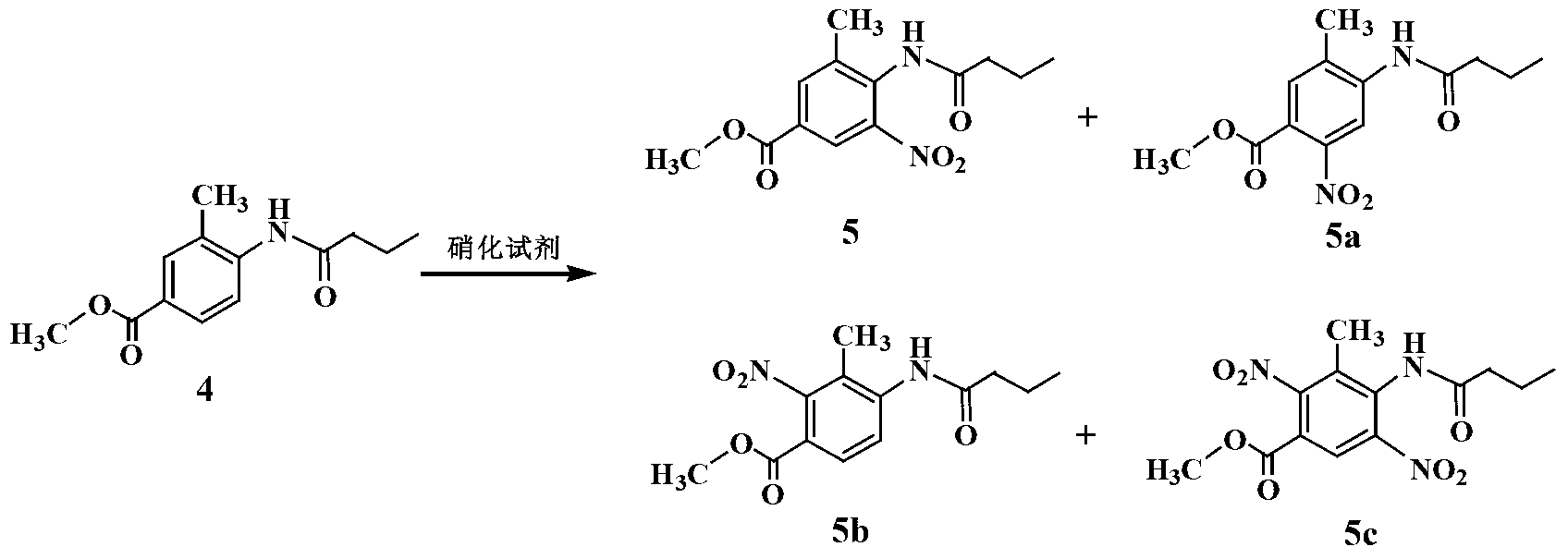
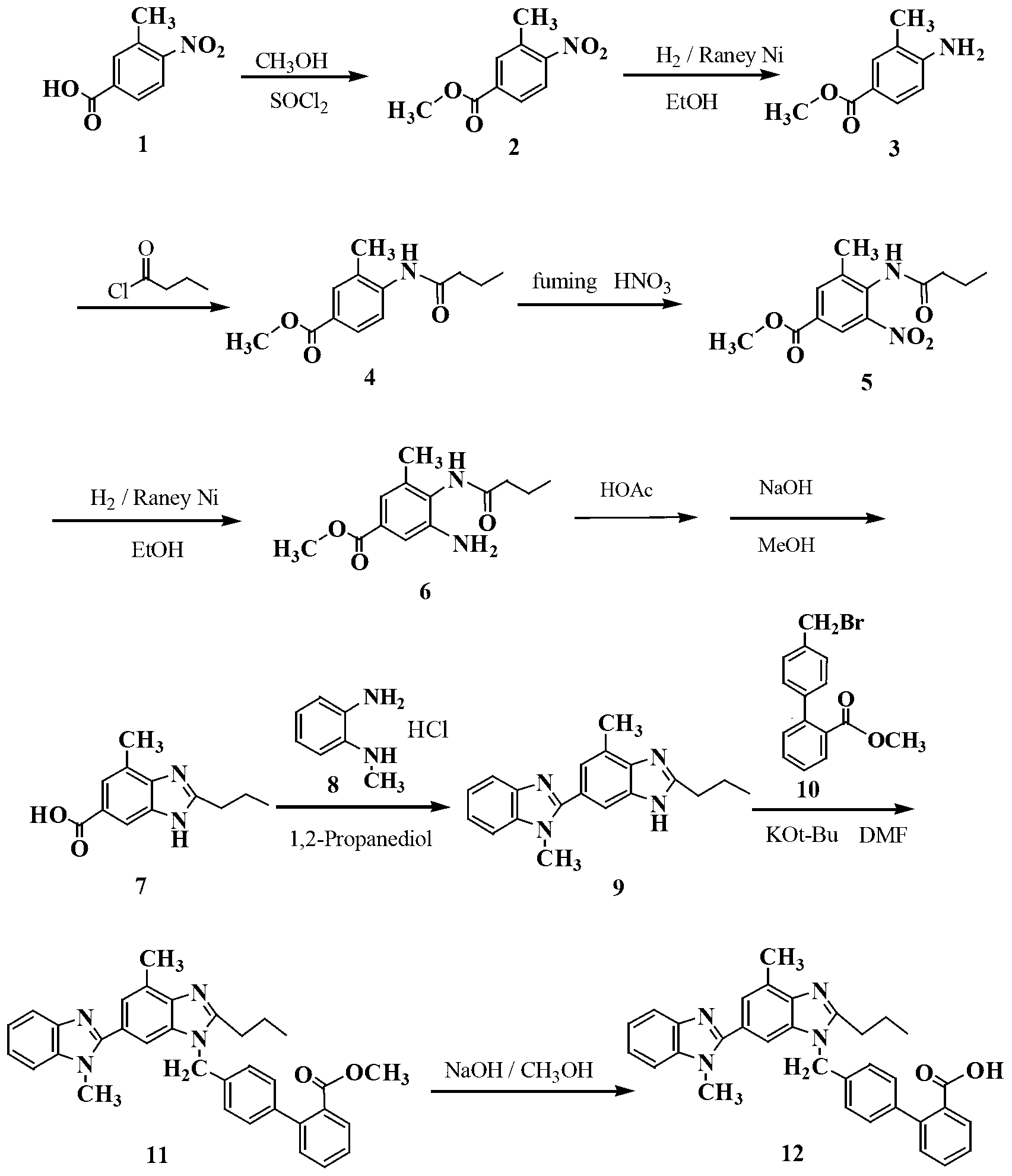
Improved telmisartan preparation process
A process improvement, telmisartan technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, large environmental pollution, cumbersome operation, etc., and achieve the effect of saving production cost, less environmental pollution, and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of methyl 3-methyl-4-nitrobenzoate (2)
[0019] Add 300g of 3-methyl-4-nitrobenzoic acid 1 (1.66mol) and 1000mL of methanol into a dry 3L three-necked flask equipped with a reflux condenser and a thermometer, and make a simple tail gas absorption device on the reflux condenser. For NaOH aqueous solution. Under electromagnetic stirring, 100 g of thionyl chloride (0.85 mol) was added dropwise with a constant pressure dropping funnel, and the temperature of the reaction solution was controlled between 30-40 ° C during the dropping process, and the reaction solution was a milky white solution at this time. The dropwise addition was completed in about 45 minutes, and the temperature was raised to 60°C. At this time, the reactant was a clear yellow solution. Stir and reflux at this temperature for 2 hours, stop heating, and cool the reaction solution slowly, and a large amount of solids precipitate out at 50°C. Continue to cool down to 30°C, add 1000mL of water ...
Embodiment 2
[0021] Preparation of 3-methyl-4-aminobenzoic acid methyl ester (3)
[0022] In a 2L hydrogenation kettle, add 1.2mL of absolute ethanol, 6.5g of Raney Ni catalyst and 130g of compound 2, pump out the air with an oil pump, and pass N 2 The air in the kettle was exhausted three times, and H was introduced into the kettle. 2 Gas to 1.2MPa. Adjust the reactor controller, raise the temperature to 80°C and observe the pressure inside the reactor. When the hydrogen pressure no longer decreases, react under this condition for 2 hours. The reactant was cooled to room temperature, the residual hydrogen in the kettle was discharged, and the reaction solution was emitted. The catalyst was removed by filtration, and the ethanol solvent was evaporated under reduced pressure to obtain 105.5 g of a white solid with a yield of 96.2%.
Embodiment 3
[0024] Preparation of methyl 3-methyl-4-n-butyramide benzoate (4)
[0025] Add 182 g (1.1 mol) of compound 3 into a 3 L three-neck flask equipped with a reflux condenser and a thermometer, and add 1.6 L of acetonitrile and 350 mL of triethylamine in sequence under stirring. Under the condition of ice-water bath, 160.5 g of n-butyryl chloride (1.5 mol) was added dropwise to the three-necked flask through a constant pressure dropping funnel, and the temperature during the dropping process was controlled not to exceed 20°C. At this time, the reaction liquid was a milky white liquid. After the dropwise addition, the temperature was slowly raised to reflux, and the solution was clear and transparent. The reaction was stirred for 2 h, cooled naturally to room temperature, and the precipitated white solid was filtered off. The acetonitrile solution was evaporated under reduced pressure, and the solid residue was washed with saturated Na 2 CO 3 The solution and water were washed se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Recrystallization yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com