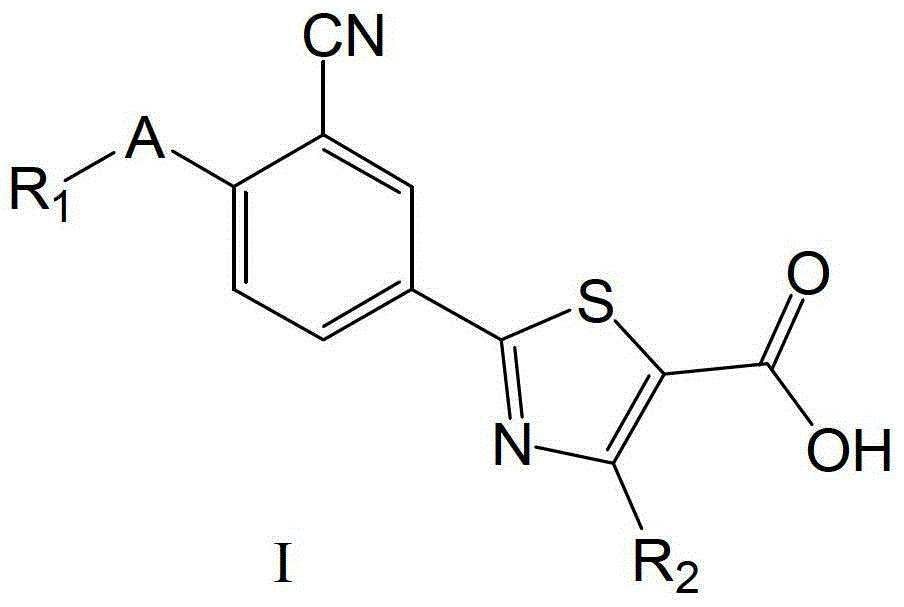
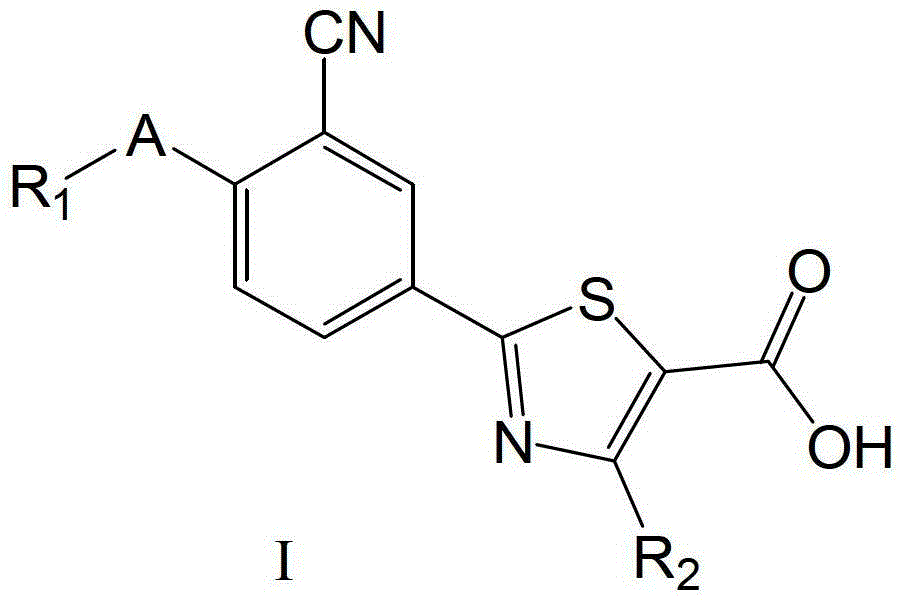
2-(3-cyano-4-alkoxy) phenyl-4-substituted thiazole-5-formic acid compound, composition as well as preparation methods and applications thereof
A technology of phenylthiazole and compound, which is applied in the field of treatment and/or prevention of hyperuricemia and gout, and can solve the problems of undiscovered thiazole-5-carboxylic acid compound composition and preparation method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0450] Example 1 Preparation of 2-(3-cyano-4-isobutoxy)phenyl-4-phenylthiazole-5-carboxylic acid (general method eight)
[0451] Add 6.1g (0.015mol) ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-phenylthiazole-5-carboxylate (intermediate 85) to a 250mL single-necked bottle, 1.9g ( 0.045mol) lithium hydroxide, 12mL water, 120mL DMF, react at 50°C for 8h, after the reaction is complete, pour the above reaction solution into 500mL water, adjust the pH value to 1 with concentrated hydrochloric acid, let stand at room temperature for 4h, suction filter, and collect The crude product was obtained by filtering the cake, and recrystallized (absolute ethanol: acetone = 1:1) to obtain 4.1 g of white powder, yield: 72.2%, mp: 199.4-199.9°C.
[0452] MS(ESI):m / z377.1[M-H] - ;IR(KBr)3429.7,2963.0,2230.3,1724.8,1652.8,1603.3,1573.6,1512.1,1485.5,1441.4,1386.5,1358.3,1327.4,1297.4,1255.9,1201.6,1179.0,1133.7,1078.8,1023.6,1002.5cm -1 ; 1 H-MNR (300MHz, DMSO-d 6 )δ(ppm):8.37(d,1H,J=2.1Hz,Ar-H),8....
Embodiment 2
[0453] Example 2 Preparation of 2-(3-cyano-4-methoxy)phenyl-4-phenylthiazole-5-carboxylic acid
[0454] According to General Method 8, using (Intermediate 86) as raw material, the crude product was recrystallized (absolute ethanol: acetone = 1:1) to obtain 3.5 g of white powder, yield: 69.4%, mp: 254.8-255.4 °C.
[0455] MS(ESI):m / z337.1[M+H] + ;IR(KBr):3442.3, 2937.9, 2361.4, 2229.7, 1678.0, 1648.4, 1603.2, 1511.1, 1484.4, 1443.9, 1427.6, 1329.3, 1285.0, 1183.8, 1167.8, 1144.2, 11033.1.1, -1 ; 1 H-MNR (300MHz, DMSO-d 6 )δ(ppm):13.47(s,1H,COOH),8.37(d,1H,J=2.1Hz,Ar-H),8.31(dd,1H,J=2.4Hz,J=9.0Hz,Ar-H ),7.81(m,2H,Ar-H),7.46(m,3H,Ar-H),7.40(d,1H,J=9.0Hz,Ar-H),4.00(s,3H,CH 3 ).
Embodiment 3
[0456] Example 3 Preparation of 2-(3-cyano-4-ethoxy)phenyl-4-phenylthiazole-5-carboxylic acid
[0457] According to General Method 8, using (Intermediate 87) as raw material, the crude product was recrystallized (absolute ethanol: acetone = 1:1) to obtain 4.4 g of white powder, yield: 73.9%, mp: 248.1-248.9 °C.
[0458] MS(ESI):m / z349.1[M-H] - ;IR(KBr):3425.6, 2988.6, 2227.1, 1688.3, 1654.9, 1603.3, 1517.4, 1486.3, 1434.0, 1392.8, 1327.2, 1292.5, 1177.9, 1144.2, 1128.3, 1041.5cm -1 ; 1 H-MNR (300MHz, DMSO-d 6 )δ(ppm):13.44(s,1H,COOH),8.35(d,1H,J=2.1Hz,Ar-H),8.27(dd,1H,J=2.4Hz,J=8.7Hz,Ar-H ),7.80(m,2H,Ar-H),7.45(m,3H,Ar-H),7.37(d,1H,J=8.7Hz,Ar-H),4.28(q,2H,J=6.9Hz ,CH 2 ),1.40(t,3H,J=6.9Hz,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com