In-situ carbon coating preparation method for secondary lithium ion battery cathode material lithium nickel phosphate
A lithium-ion battery and cathode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as rare secondary lithium-ion batteries, and achieve improved conductivity and active material utilization, uniform particle size distribution, The effect of improving cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
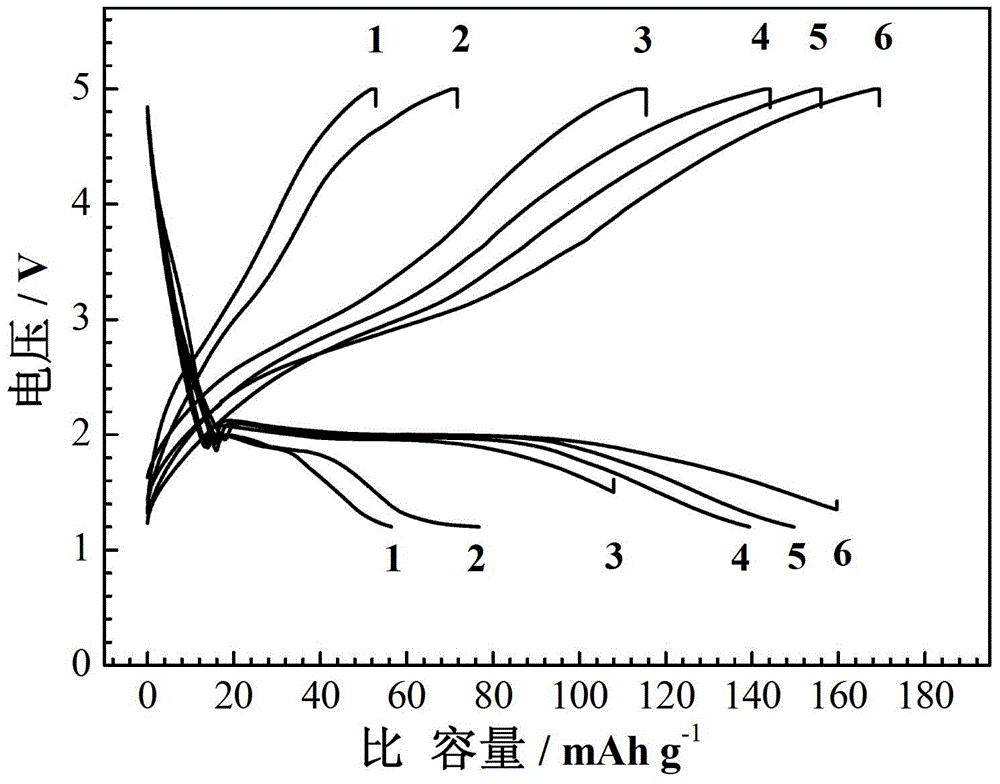
Image
Examples
Embodiment 1
[0025] (1) Will (NH 4 ) 3 PO 4 ·3H 2 O, NiSO 4 ·6H 2 O, LiOH·H 2 O was fully dissolved in benzyl alcohol aqueous solution (benzyl alcohol accounted for 50% by volume) according to the molar ratio of 1:1:3, and magnetically stirred for 30 minutes;
[0026] (2) Pour the fully stirred benzyl alcohol solution into a stainless steel reactor lined with polytetrafluoroethylene for hydrothermal reaction. The hydrothermal reaction temperature is 150°C and the hydrothermal time is 12 hours;
[0027] (3) Suction filter the product obtained by the hydrothermal reaction, and wash the impurities contained in it with distilled water and absolute ethanol;
[0028] (4) Put the cleaned material into a blast drying oven at 80°C to dry;
[0029] (5) Fully grind the dried powder, put it into a tube furnace, pretreat at 400°C for 2h in an argon atmosphere, and then calcinate at 750°C for 2h to obtain the final product;
[0030] (6) Preparation of electrode sheets and battery assembly: The e...
Embodiment 2
[0033] (1) Will (NH 4 ) 3 PO 4 ·3H 2 O, NiSO 4 ·6H2 O, LiOH·H 2 O, glucose is fully dissolved in distilled water according to the molar ratio of 1:1:3:0.5, and magnetically stirred for 30min;
[0034] (2) Pour the fully stirred benzyl alcohol solution into a stainless steel reactor lined with polytetrafluoroethylene for hydrothermal reaction. The hydrothermal reaction temperature is 150°C and the hydrothermal time is 12 hours;
[0035] (3) Suction filter the product obtained by the hydrothermal reaction, and wash the impurities contained in it with distilled water and absolute ethanol;
[0036] (4) Put the cleaned material into a blast drying oven at 80°C to dry;
[0037] (5) Fully grind the dried powder, put it into a tube furnace, pretreat at 400°C for 2h in an argon atmosphere, and then calcinate at 750°C for 2h to obtain the final product;
[0038] (6) Preparation of electrode sheets and battery assembly: The electrode paste is made of binder LA132, Ketjen black KB,...
Embodiment 3
[0041] (1) Will (NH 4 ) 3 PO 4 ·3H 2 O, NiSO 4 ·6H 2 O, LiOH·H 2 O. Glucose is fully dissolved in benzyl alcohol aqueous solution (benzyl alcohol accounts for 50% by volume) according to the molar ratio of 1:1:3:0.5, and magnetically stirred for 30 minutes;
[0042] (2) Pour the fully stirred benzyl alcohol solution into a stainless steel reactor lined with polytetrafluoroethylene for hydrothermal reaction. The hydrothermal reaction temperature is 180°C and the hydrothermal time is 12 hours;
[0043] (3) Suction filter the product obtained by the hydrothermal reaction, and wash the impurities contained in it with distilled water and absolute ethanol;
[0044] (4) Put the cleaned material into a blast drying oven at 80°C to dry;
[0045] (5) Fully grind the dried powder, put it into a tube furnace, pretreat at 400°C for 2h in an argon atmosphere, and then calcinate at 750°C for 2h to obtain the final product;
[0046] (6) Preparation of electrode sheets and battery asse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com