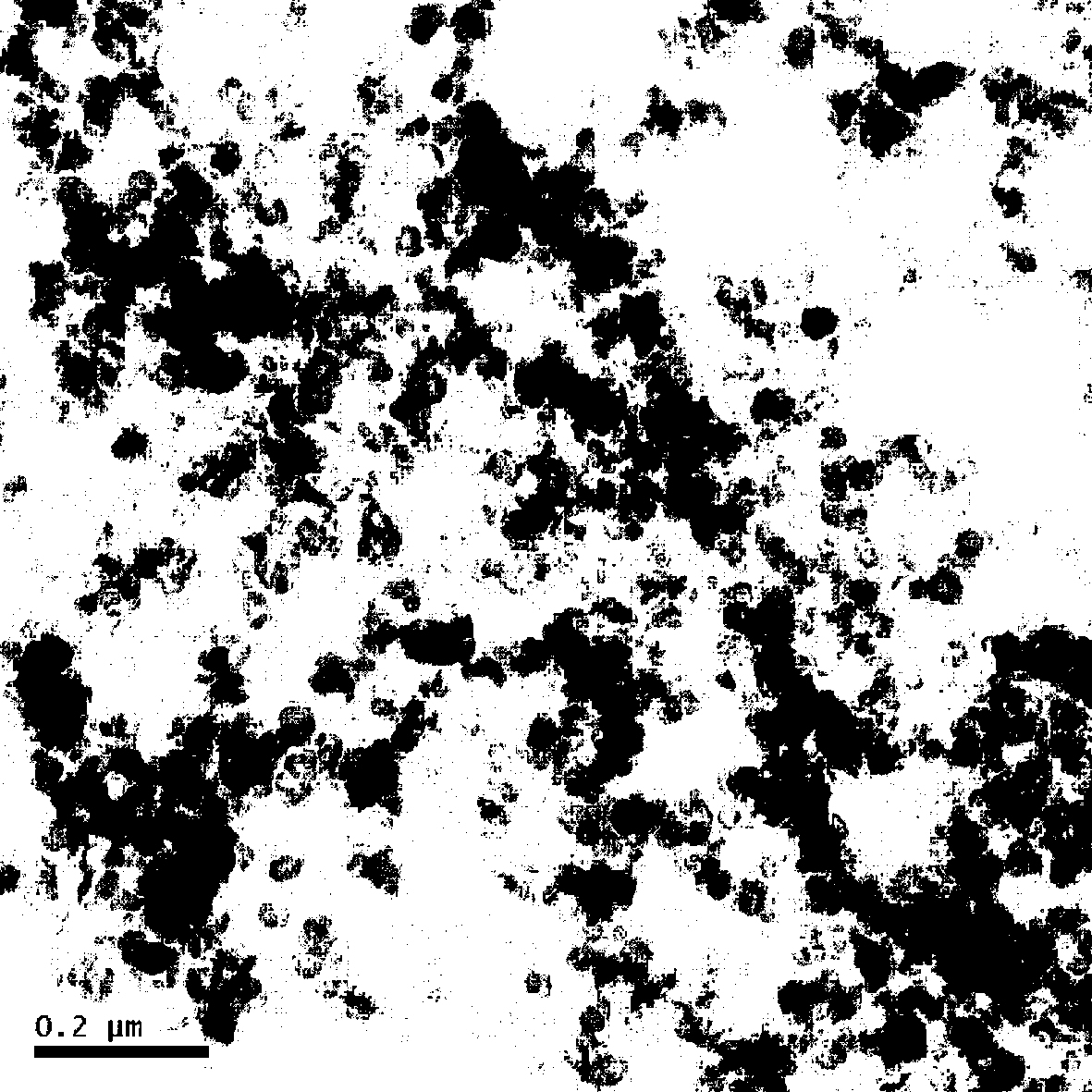
Preparation method of carbon-coated lithium iron phosphate material with polystyrene microspheres and polyethylene glycol as carbon sources
A technology of carbon-coated lithium iron phosphate and polystyrene microspheres, which is applied to electrical components, battery electrodes, circuits, etc., can solve the problems of low electronic conductivity of lithium iron phosphate and slow diffusion of lithium ions, and achieve safety performance and Improved battery performance, low cost, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Mix 2% NaOH solution with an equal volume of styrene, separate with a separatory funnel, take the supernatant, and repeat this three times to remove the polymerization inhibitor in the styrene solution to obtain treated styrene monomer;
[0022] (2) Dissolve sodium lauryl sulfate and potassium persulfate in 90ml water mixture according to the mass ratio of 2:1;
[0023] (3) in N 2 Stir for 20 minutes under protection, then heat up to 50°C, heat for 10 minutes, then slowly add the treated styrene monomer dropwise, and react for 24 hours to obtain polystyrene microspheres;
[0024] (4) The obtained polystyrene microspheres and polyethylene glycol are mixed and dissolved in deionized water at a mass ratio of 1:1, and then lithium acetate, iron nitrate and dihydrogen phosphate are added at a mass ratio of 2:3:1 Ammonium, forming a mixed gel;
[0025] (5) Stir the resulting gel at 60 °C for 2 hours,
[0026] (6) Dry in an oven at a constant temperature of 100°C for 1...
Embodiment 2
[0032] (1) Mix 5% NaOH solution with an equal volume of styrene, separate with a separatory funnel, take the supernatant, repeat this three times, remove the polymerization inhibitor in the styrene solution, and obtain treated styrene monomer;
[0033] (2) Dissolve sodium lauryl sulfate and potassium persulfate in 90ml water mixture according to the mass ratio of 3:2;
[0034] (3) in N 2 Stir for 40 minutes under protection, then heat up to 90°C, heat for 10 minutes, then slowly add the treated styrene monomer dropwise, and react for 24 hours to obtain polystyrene microspheres;
[0035] (4) The obtained polystyrene microspheres and polyethylene glycol are mixed and dissolved in deionized water at a mass ratio of 4:1, and then lithium acetate, iron nitrate and dihydrogen phosphate are added at a mass ratio of 1:2:1 Ammonium, forming a mixed gel;
[0036] (5) Stir the resulting gel at 80 °C for 3 hours,
[0037] (6) Dry in an oven at a constant temperature of 150°C for 12 ho...
Embodiment 3
[0043] (1) Mix 10% NaOH solution with equal volume of styrene, separate with a separatory funnel, take the supernatant, repeat this three times, remove the polymerization inhibitor in the styrene solution, and obtain treated styrene monomer;
[0044] (2) Weigh sodium lauryl sulfate and potassium persulfate at a mass ratio of 3:1 and dissolve them in 90ml of water mixture;
[0045] (3) in N 2Stir for 60 minutes under protection, then heat up to 70°C, heat for 10 minutes, then slowly add the treated styrene monomer dropwise, and react for 24 hours to obtain polystyrene microspheres;
[0046] (4) Mix and dissolve the obtained polystyrene microspheres and polyethylene glycol in deionized water at a mass ratio of 2:1, and then add lithium acetate, iron nitrate and dihydrogen phosphate at a mass ratio of 2:4:2 Ammonium, forming a mixed gel;
[0047] (5) Stir the resulting gel at 60-90 °C for 23 hours,
[0048] (6) Dry in an oven at a constant temperature of 120°C for 12 hours; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com