Bi2WO6 modified TiO2 nanobelt photocatalyst, preparation method and application thereof
A photocatalyst and nanobelt technology, applied in the field of nanophotocatalysis, can solve the problems of difficult recycling and reuse, secondary pollution, easy agglomeration, etc., and achieve the effects of easy recycling and reuse, low band gap width and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Pretreatment of titanium sheets: The titanium sheets with a size of 25mm×50mm×0.25mm were ultrasonically washed with detergent powder, deionized water, acetone, alcohol, and deionized water for 5 minutes to remove surface pollutants, and then the titanium sheets Put it into a mixed acid solution of hydrofluoric acid, nitric acid and deionized water with a volume ratio of 1:1:4 for chemical polishing to remove surface impurities, and then dry it at room temperature under nitrogen protection.
[0036] Titanium dioxide nanoarrays (TiO 2 nanobelt): Put the pretreated titanium sheet into a 50mL hydrothermal reactor lined with polytetrafluoroethylene, add 35mL of sodium hydroxide solution with a concentration of 3mol / L, and place it at 200°C Under the hydrothermal reaction for 48h, naturally cooled to room temperature after the reaction, took out and rinsed with deionized water.
[0037] TiO 2 Preparation of nanobelt photocatalyst: Put the titanium dioxide nanoarray with ti...
Embodiment 2
[0040] Pretreatment of titanium sheet and TiO 2 The preparation of the nanobelt is the same as in Example 1.
[0041] 0.01mol of analytically pure Bi(NO 3 ) 3 Dissolved in 5mL of glacial acetic acid, additionally 0.005mol of analytically pure Na 2 WO 4 Dissolve in 90mL deionized water, then mix the two solutions together, stir for 2h to form a white suspension (and adjust the pH of the suspension to 3 with glacial acetic acid).
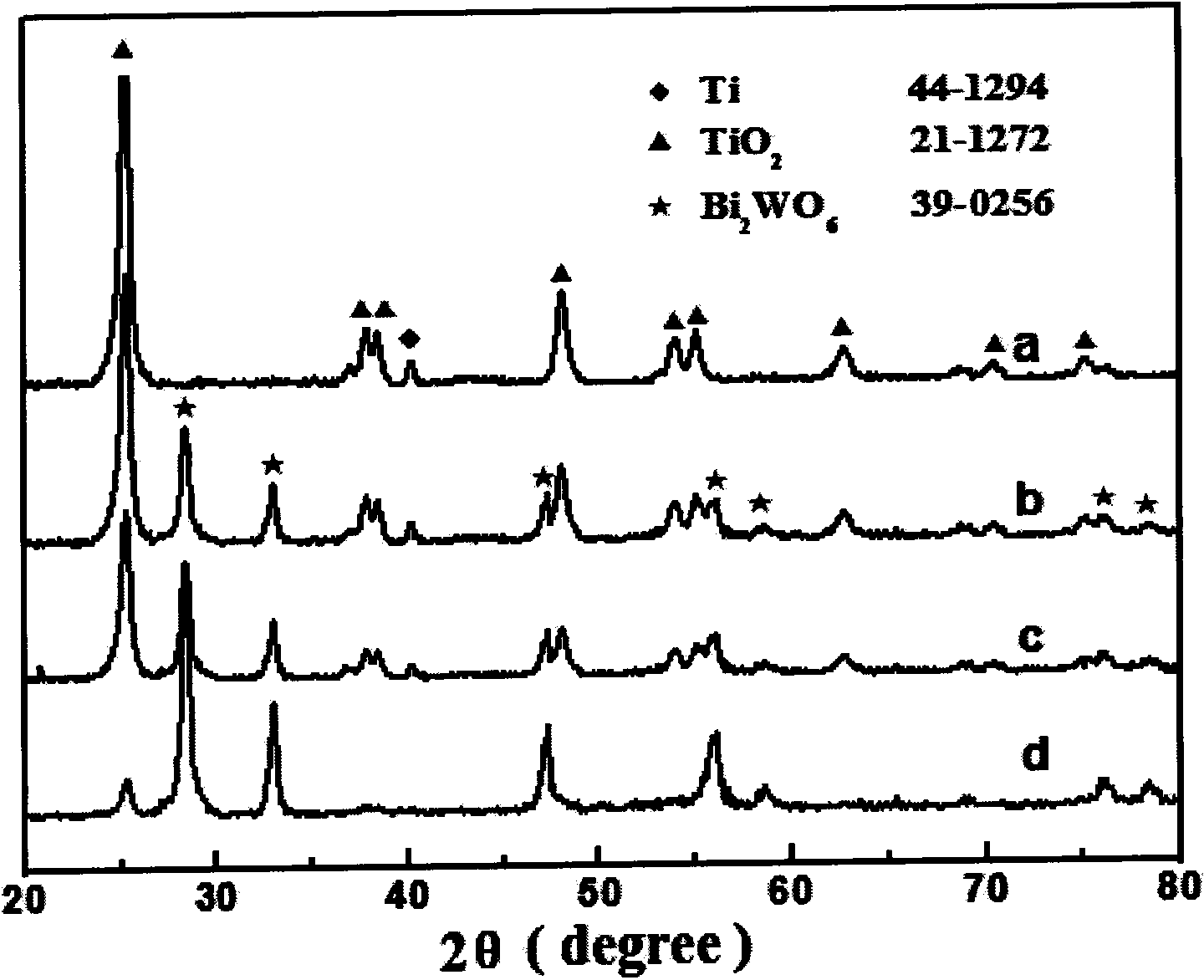
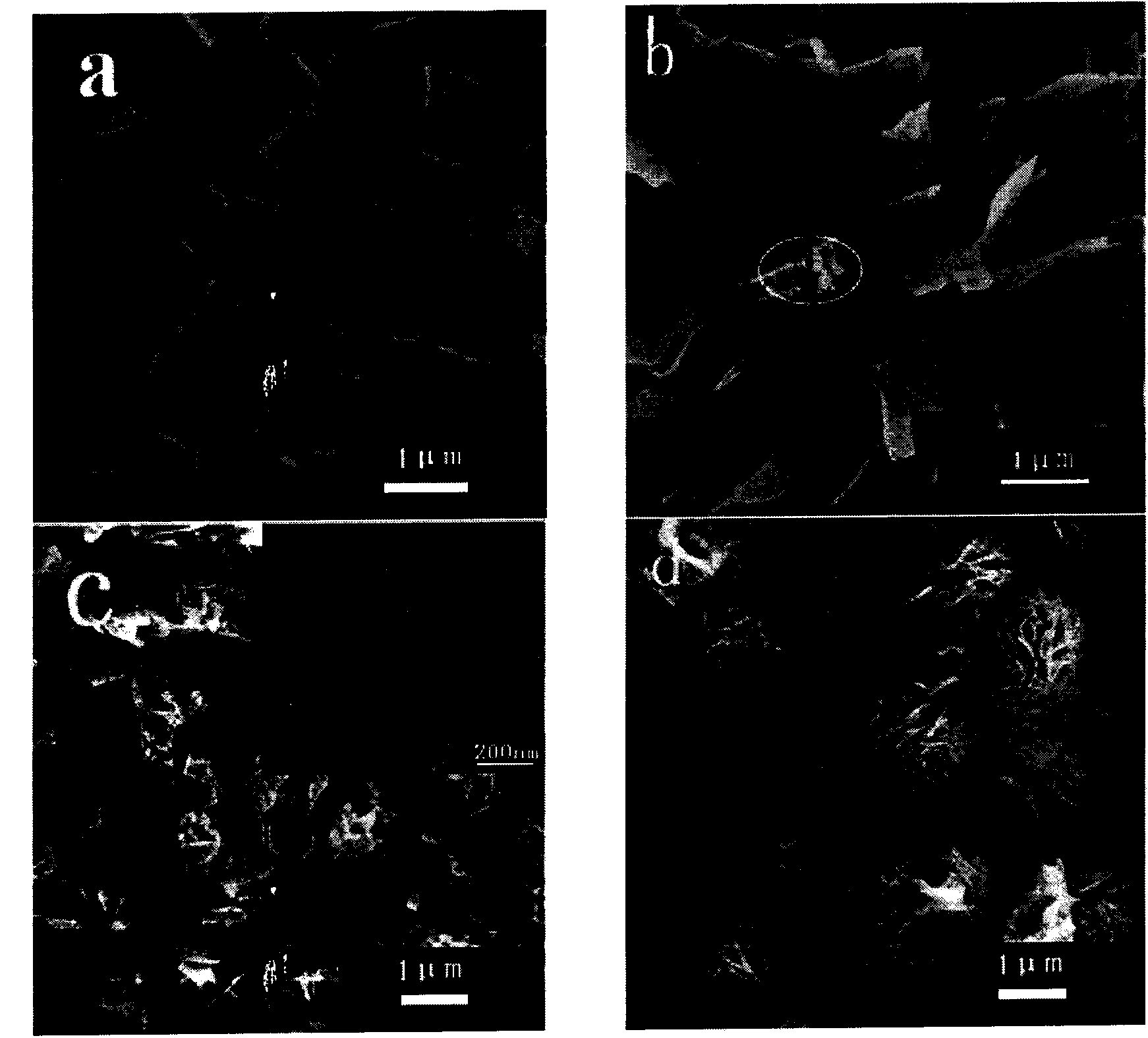
[0042] TiO 2 The nanobelts and 35mL white suspension were placed in a 50mL hydrothermal reaction kettle lined with polytetrafluoroethylene, and hydrothermally reacted at 140°C for 20h. After cooling to room temperature naturally, they were taken out and rinsed with deionized water. Dry at room temperature under nitrogen protection. And calcined at 400°C for 3h, you can get Bi 2 WO 6 Modified TiO 2 nanobelt. For its XRD diagram and SEM diagram, please refer to figure 1 and 2 .
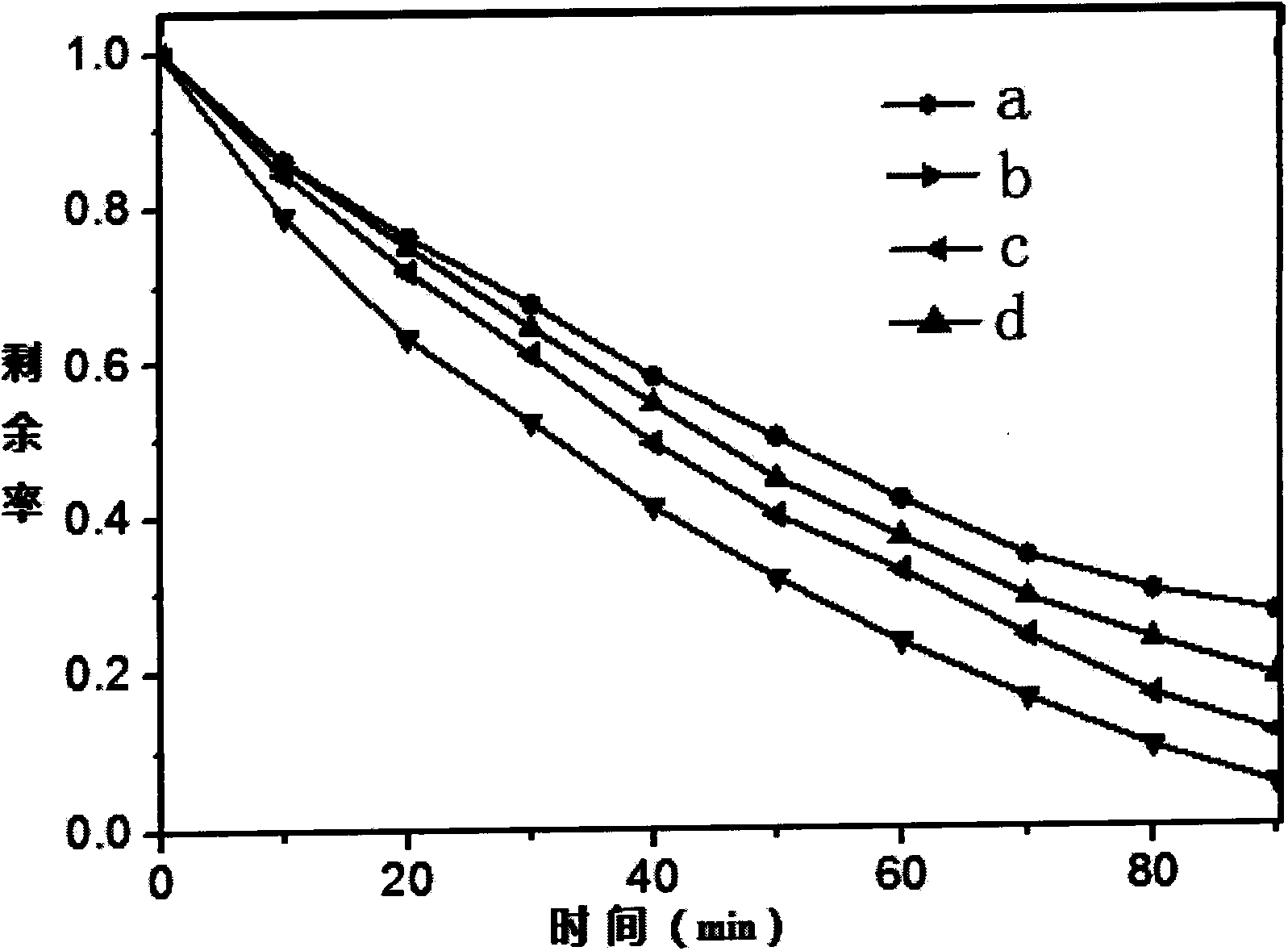
[0043] like image 3 As shown, under the irradiation of 350W...
Embodiment 3
[0045] Pretreatment of titanium sheet and TiO 2 The preparation of the nanobelt is the same as in Example 1.
[0046] 0.02mol of analytically pure Bi(NO 3 ) 3 Dissolved in 5mL of glacial acetic acid, additionally 0.01mol of analytically pure Na 2 WO 4 Dissolve in 90mL deionized water, then mix the two solutions together, stir for 2h to form a white suspension (and adjust the pH of the suspension to 3 with glacial acetic acid).
[0047] TiO 2 The nanobelts and 35mL white suspension were placed in a 50mL hydrothermal reaction kettle lined with polytetrafluoroethylene, and hydrothermally reacted at 140°C for 20h. After cooling to room temperature naturally, they were taken out and rinsed with deionized water. Dry at room temperature under nitrogen protection. And calcined at 400°C for 3h, you can get Bi 2 WO 6 Modified TiO 2 nanobelt. For its XRD diagram and SEM diagram, please refer to figure 1 and 2 , photocatalytic cycle degradation diagram please refer to Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com