Method for producing 1,4-butanediol, tetrahydrofuran, gamma-butyrolactone and butanol
A technology of tetrahydrofuran and butanediol, applied in chemical instruments and methods, preparation of organic compounds, chemical industry, etc., can solve problems such as loss, complicated operation, catalyst loss, etc., to reduce the number of replacements, simplify engineering operations, and avoid distribution. uneven effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
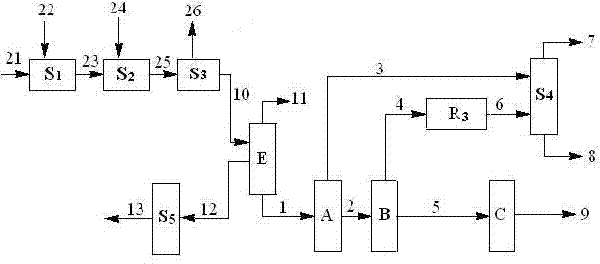
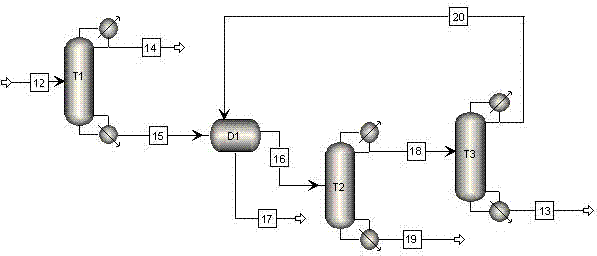
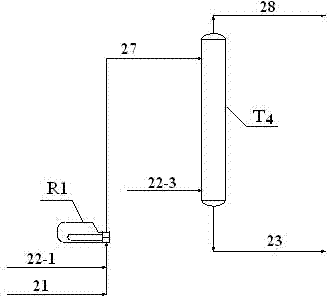
[0042] use figure 1 , figure 2 and Figure 4 The process shown, maleic anhydride 21 and methanol 22 enter the esterification unit R 1 , After the reaction, a stream 23 containing dimethyl maleate is obtained. Wherein, the maleic anhydride stream 21 and the first methanol stream 22-1 enter the monoesterification reactor R 1 , the reaction gives stream I containing monomethyl maleate. Stream I and the second methanol stream 22-2 enter the double esterification fixed bed reactor R from the bottom 2 , contact with the catalyst, and the stream 27 (i.e. stream II) containing monomethyl maleate and dimethyl maleate is obtained at the top of the tower; wherein, 85% by weight of monomethyl maleate in stream I is converted into maleic acid Acid dimethyl ester. Stream II enters the double-esterification catalytic distillation reaction tower T4 from the top, and the third methanol 22-3 enters the double-esterification catalytic distillation reaction tower T4 from the bottom. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com