Conjugated metal polymer optoelectronic materials with functionalized polar side chain groups and their applications
A technology of metal polymers and polar side chains, applied in photovoltaic power generation, circuits, electrical components, etc., can solve the problems that are rarely reported and the conjugated metal polymers have not attracted attention, and achieve reduced thickness dependence and good electronic performance. Effect of transport/extraction properties, high migration properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Conjugated metal polymer photoelectric material with functionalized polar side chain group poly[2,7-diethynyl-9,9'-bis(6-N,N-diethylamino-hexyl)fluorene- Preparation of mercury] (referred to as PFE6N-Hg)
[0047] The synthetic route is as follows:
[0048]
[0049] (1) 2,7-diethynyl-9,9'-bis(6-bromo-hexyl)fluorene (monomer 4) is prepared according to the method disclosed in the literature [J.Phys.Chem.B2008,112,9295] .
[0050] (2) Synthesis of 2,7-diethynyl-9,9'-bis(6-N,N-diethylamino-hexyl)fluorene (monomer FE6N)
[0051] Add the raw material 2,7-diethynyl-9,9'-bis(6-bromo-hexyl)fluorene (4.74g, 9.0mmol) into the reaction flask, add 300mL N,N-dimethylformamide (DMF ) Dissolve the raw materials, then add diethylamine (15mL), heat to reflux for 12h under the protection of argon, pour the reaction solution into ice water after cooling, extract with dichloromethane, concentrate and pass the concentrate to the column to obtain The product was 4.38 g, and the yield was 85%.
[00...
Embodiment 2
[0059] Conjugated metal polymer photoelectric material with functionalized polar side chain group poly[2,7-diethynyl-9,9'-bis(3-N,N-diethylamino-propyl) fluorene -Mercury] (referred to as PFE3N-Hg) preparation
[0060] The synthetic route is as follows:
[0061]
[0062] (1) 2,7-Dibromo-9,9'-bis(3-bromo-propyl)fluorene (monomer 5)
[0063] Add the raw material 2,7-dibromofluorene (13.0g, 40mmol) into the reaction flask, add 150mL 1,3-dibromopropane, stir, add 20mL50% sodium hydroxide aqueous solution, 0.5g tetrabutylammonium bromide, The reaction was carried out at 50-60°C for 12 hours under the protection of argon. After cooling, the reaction solution was poured into ice water, extracted with dichloromethane and concentrated, and the concentrate was passed through a column to obtain 18.1 g of product with a yield of 80%.
[0064] The NMR data of the product are as follows:
[0065] 1 H NMR(300MHz, CDCl 3 , δ, ppm) 7.55-7.49 (m, 6H), 3.15-3.11 (t, 4H, J=6.54Hz), 2.16-2.11 (m, 4H), 1.1...
Embodiment 5
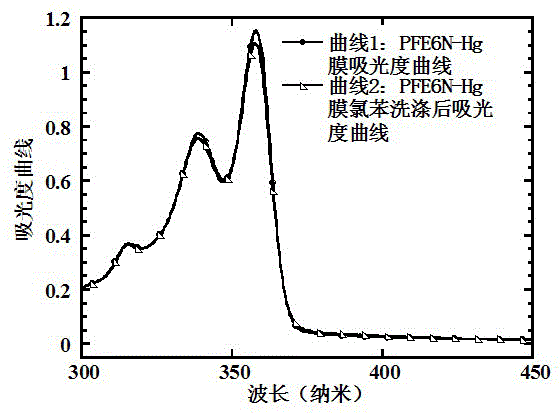
[0093] Taking the polymer PFE6N-Hg synthesized in Example 1 as an example, it is shown that this type of polymer has anti-solvent (chlorobenzene) elution performance and can use orthogonal solvents to prepare multilayer organic / polymer solar cell devices.
[0094] Dissolve PFE6N-Hg in 1,4-dioxane, filter it with a 0.45μm organic filter membrane, and spin-coat on a common glass plate to form a film with a thickness of about 20nm. Use the UV tester (HP8453spectrophotometer) produced by HP to measure the absorbance of PFE6N-Hg after film formation, which corresponds to figure 1 Curve 1 in. Then, the PFE6N-Hg film was eluted with chlorobenzene, and the absorbance of the eluted PFE6N-Hg film was tested by UV, corresponding to figure 1 The curve in 2. By observing that the absorbance of PFE6N-Hg film does not decrease after elution with chlorobenzene, it has excellent anti-solvent elution performance.
[0095] figure 1 A graph of the ultraviolet-visible light (UV) absorbance curve of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com