Cnidium coumarin is used to prepare the purposes of antifungal drug synergist product
An antifungal drug, a technology of coumarin, applied in the field of medicine, can solve the problem of no public report on the synergistic antifungal effect, achieve good application prospect, wide application prospect, and improve the effect of antifungal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
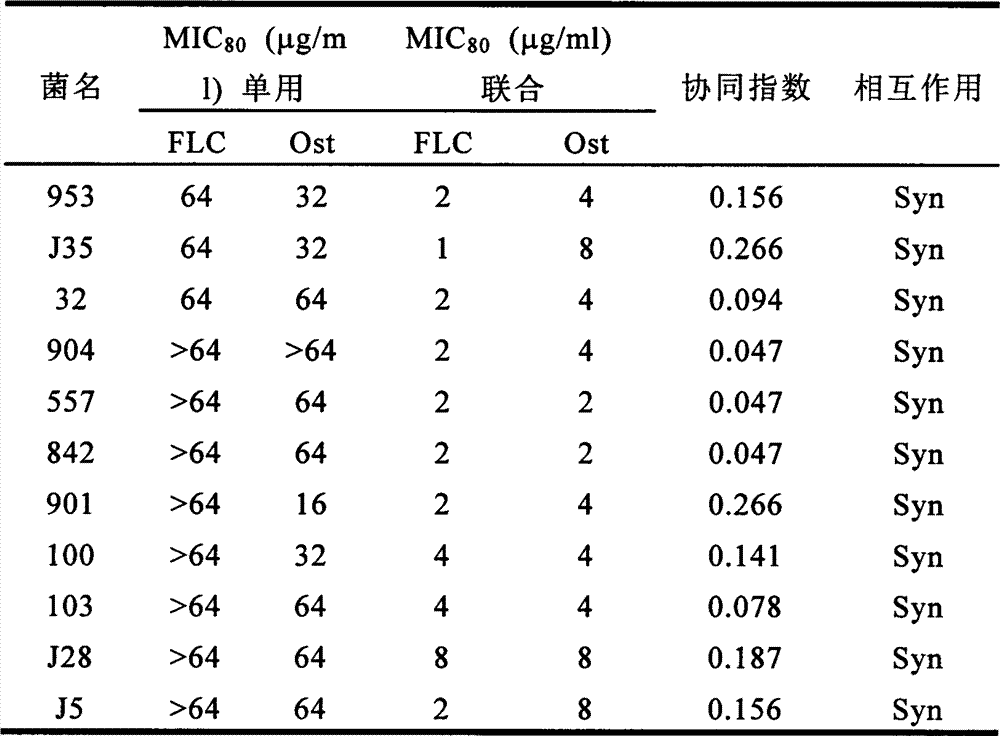
[0011] Example 1: The effect of the combination of methoxycarvacrol and fluconazole on different clinical fungal strains
[0012] Materials and methods
[0013] 1. Test drug:
[0014] Methoxyparscreol: provided by Xi'an Luquan Biotechnology Co., Ltd., prepared with DMSO.
[0015] Fluconazole injection: Pfizer Pharmaceutical Co., Ltd., batch number 9149102.
[0016] All reagents were stored at -20°C. Before the experiment, the drug was taken out and placed in a 35°C incubator to melt, mixed well, and pharmacodynamic tests were carried out respectively.
[0017] 2. Strains:
[0018] Clinical strains: Drug-resistant Candida albicans (100, J35, 32, 557, 842, 901, 103, J28, J5, 904, 953), all provided by the Fungi Department of Shanghai Changhai Hospital, collected from clinical samples of different departments of Changhai Hospital , and were identified morphologically and biochemically.
[0019] All the experimental strains were activated on sandcastle dextrose agar (SDA) me...
Embodiment 2
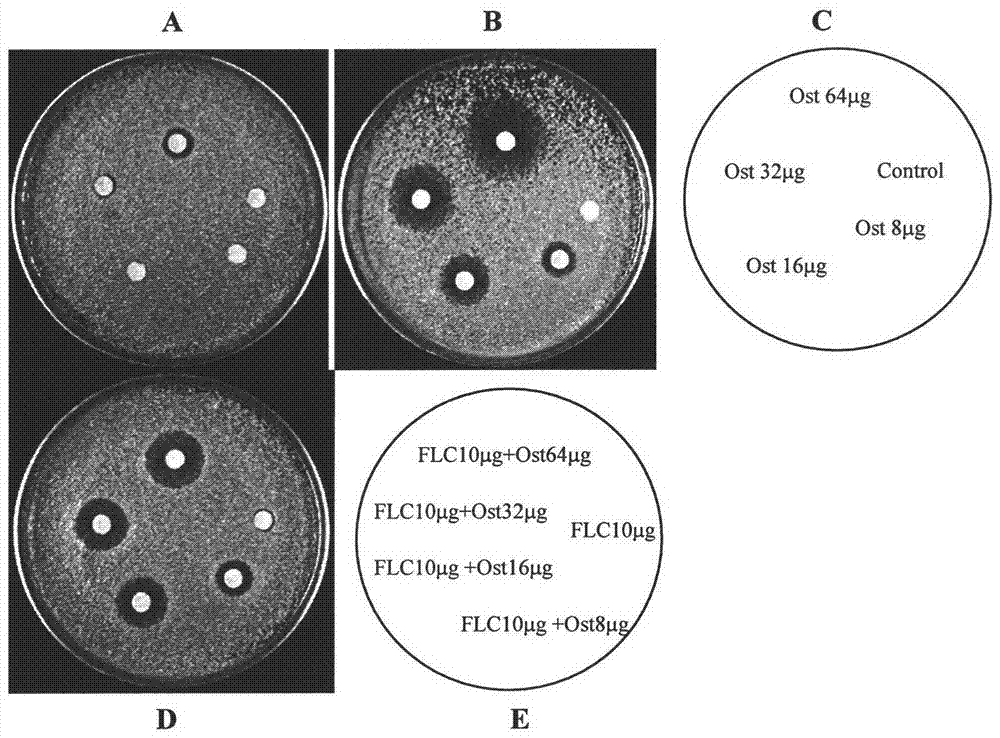
[0044] Example 2: Diffusion experiment on agar plate in combination with methoxyparvacrol and fluconazole
[0045] Materials and methods
[0046] Strains: Clinical drug-resistant Candida albicans No. 0304103, provided by the Fungi Department of Changhai Hospital, and identified by morphology and biochemistry.
[0047] Drug: fluconazole injection (Diflucan, Diflucan) is produced by Pfizer Biopharmaceutical Co., Ltd. of the United States. Methoxyparserol (osthol) was provided by Xi'an Luquan Biotechnology Co., Ltd. and prepared in DMSO.
[0048] Reagents: YEPD culture fluid, YEPD solid medium, DMSO, 0.9% physiological saline, sterile white paper (diameter 6mm).
[0049]YEPD solid medium: 10g of yeast extract, 20g of peptone, 20g of glucose, 20g of agar, add 900ml of triple distilled water to dissolve, adjust the volume to 1000ml with triple distilled water, autoclave (121°C, 15min), spread the liquid while hot Store in a sterile petri dish (diameter 90mm) at 4°C for later use...
Embodiment 3
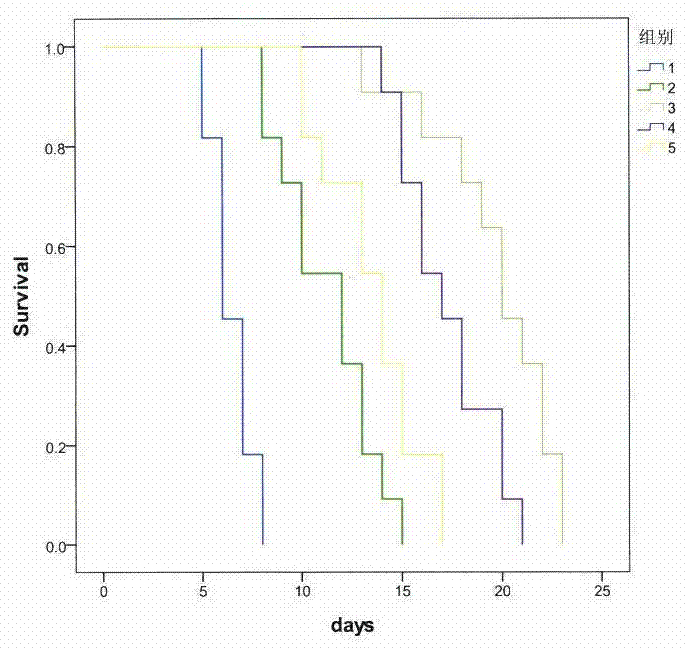
[0054] Example 3: The protective effect of combined use of methoxyparvacrol and fluconazole on systemic fungal infection in mice
[0055] Test design:
[0056] 50 ICR mice were injected into the tail vein with 5×10 6 CFU / ml logarithmic growth phase Candida albicans suspension 0.2ml, replicate systemic mouse deep fungal infection model, and randomly divide into the following 5 groups: model group, fluconazole 0.5mg / ml group, fluconazole Azole 0.5mg / ml+methoxyparscreol 75mg / ml group, fluconazole 0.5mg / ml+methoxyparscreol 150mg / ml group, fluconazole 0.5mg / ml+methoxyparscreol 300mg / ml Group.
[0057] Two hours after modeling, the drug was administered by intragastric administration, with a volume of 0.2ml / 10g, once a day for four consecutive days. The mice were observed for death within 30 days after the start of administration.
[0058] statistical methods:
[0059] Kaplan-Meier survival analysis, statistical software SPSS17.0.
[0060] Experimental results
[0061] The ef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com