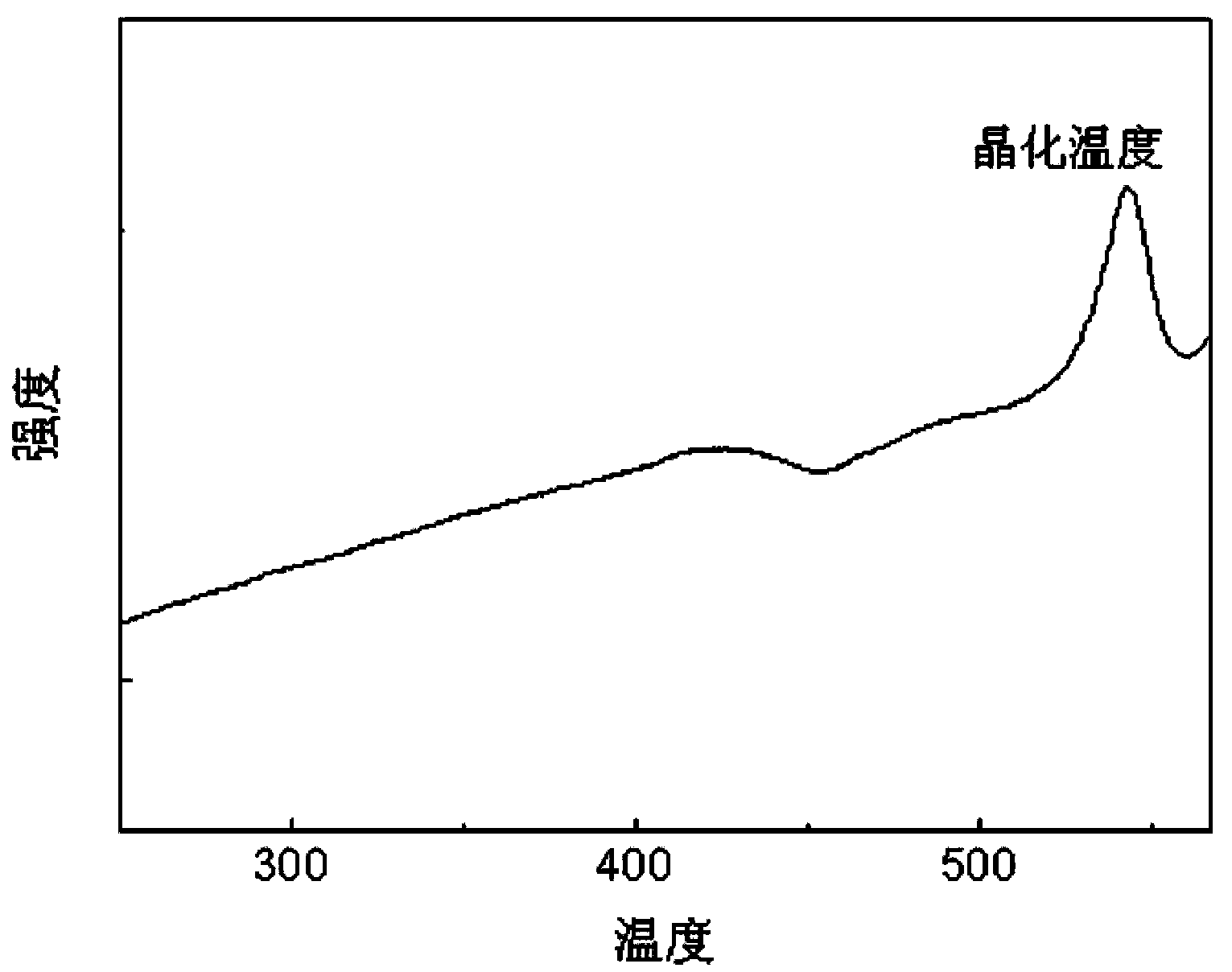
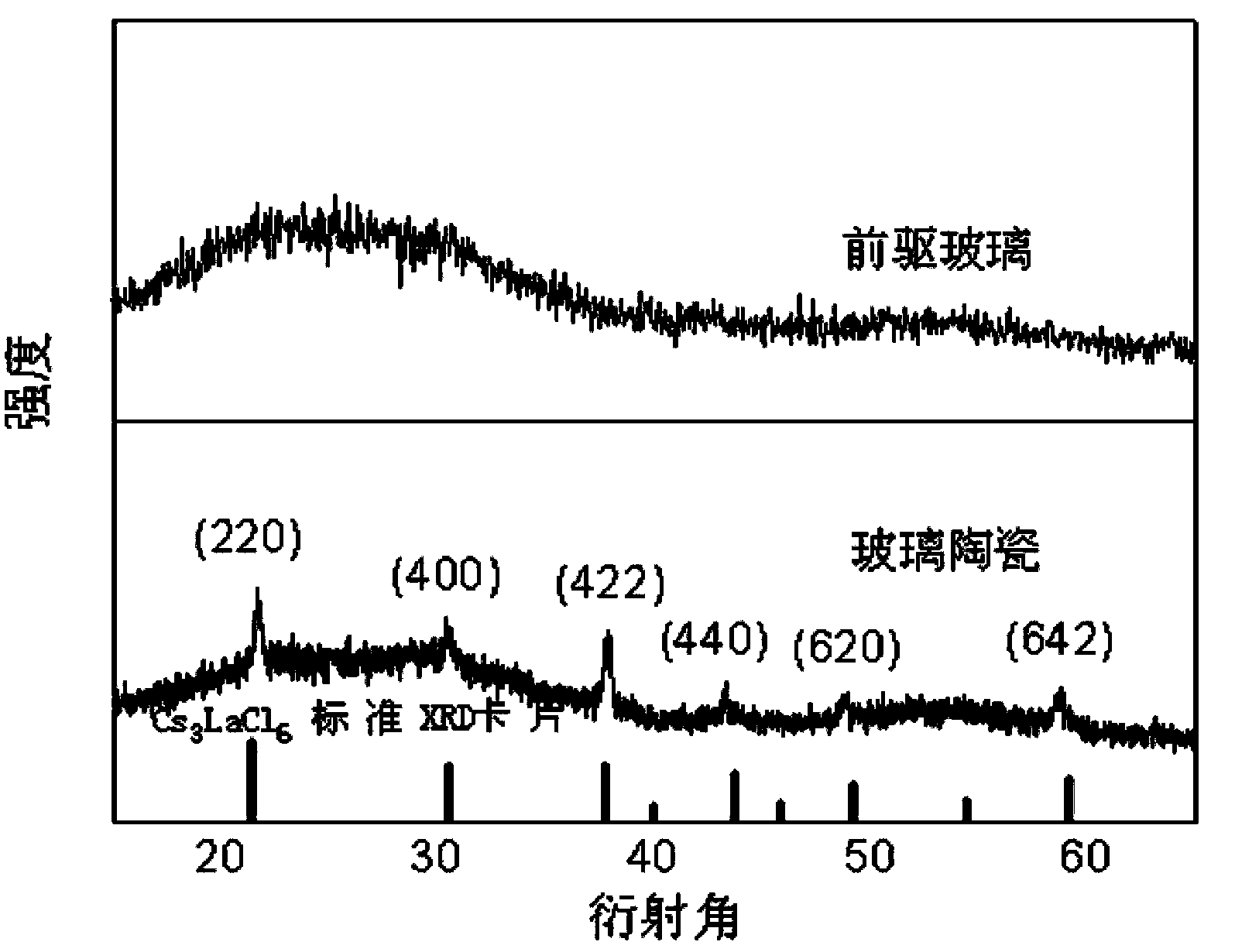
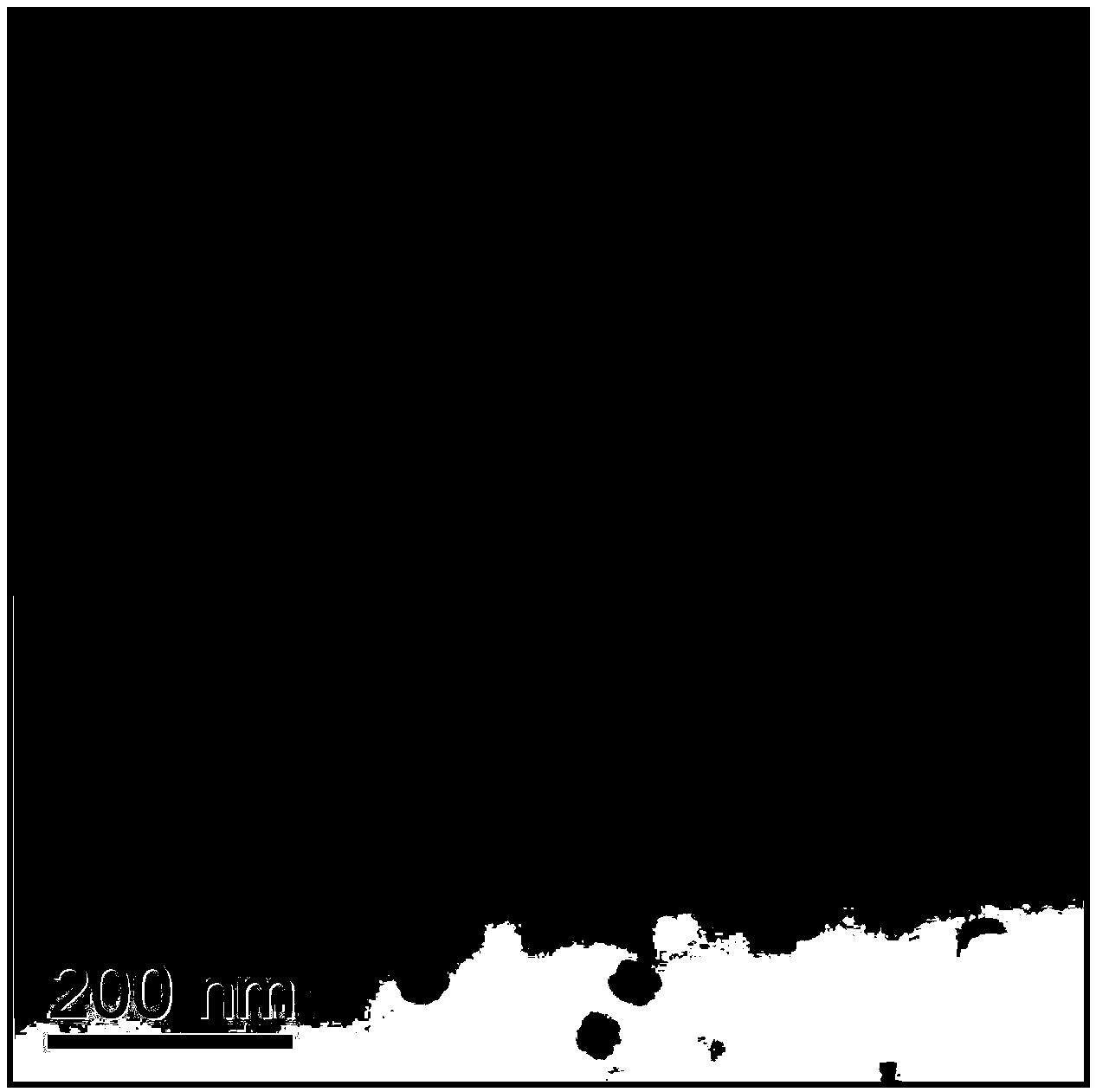
Cs3LaCl6 nanocrystalline-containing transparent chalcohalide glass ceramic and its preparation
A technology of sulfur halide glass and glass ceramics, applied in the field of optical materials, can solve the problems of difficult preparation, easy water absorption, low phonon energy, etc., and achieves low cost, simple preparation process, excellent near-infrared down-transfer and visible up-conversion luminescence. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Example 1: High-purity germanium powder (5N), gallium sulfide (4N), sulfur powder (5N), lanthanum sulfide (4N), lanthanum chloride (4N), cesium chloride (3N), neodymium sulfide (4N) Or erbium sulfide (4N) powder raw material according to 45GeS 2 -30Ga 2 S 3 -5.85La 2 S 3 -4LaCl 3 -15CsCl-0.15Nd 2 S 3 (Er 2 S 3 ) after accurately weighing the molar components, place them in a quartz tube, connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and medicine; when the vacuum degree reaches 10 -3 At mbar, seal the quartz tube with an oxyhydrogen flame; put the sealed quartz tube into a swing furnace, heat up to 800°C and start to swing, and keep it warm for 12 hours to melt it; then, take out the quartz tube and insert it vertically into the water After quenching for a few seconds, a block of sulfur halide glass can be obtained. The precursor glass can be obtained by putting the sulfur halide glass into a resi...
example 2
[0017] Example 2: High-purity germanium powder (5N), gallium sulfide (4N), sulfur powder (5N), lanthanum sulfide (4N), lanthanum chloride (4N), cesium chloride (3N), neodymium sulfide (4N) Or erbium sulfide (4N) powder raw material according to 40GeS 2 -35Ga 2 S 3 -6.85La 2 S 3 -4LaCl 3 -14CsCl-0.15Nd 2 S 3 (Er 2 S 3 ) after accurately weighing the molar components, place them in a quartz tube, connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and medicine; when the vacuum degree reaches 10 -3 At mbar, seal the quartz tube with an oxyhydrogen flame; put the sealed quartz tube into a swing furnace, heat up to 950°C and start to swing, and keep it warm for 16 hours to melt it; then, take out the quartz tube and insert it vertically into the water After quenching for a few seconds, a block of sulfur halide glass can be obtained. The precursor glass can be obtained by putting the sulfur halide glass into a resi...
example 3
[0018] Example 3: High-purity germanium powder (5N), gallium sulfide (4N), sulfur powder (5N), lanthanum sulfide (4N), lanthanum chloride (4N), cesium chloride (3N), neodymium sulfide (4N) Or erbium sulfide (4N) powder raw material according to 56GeS 2 -20Ga 2 S 3 -3.85La 2 S 3 -4LaCl 3 -16CsCl-0.15Nd 2 S 3 (Er 2 S 3 ) after accurately weighing the molar components, place them in a quartz tube, connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and medicine; when the vacuum degree reaches 10 -3 At mbar, seal the quartz tube with a hydrogen-oxygen flame; put the sealed quartz tube into a swing furnace, heat up to 1000°C and start to swing, and keep it warm for 24 hours to melt it; then, take out the quartz tube and insert it vertically into the water After quenching for a few seconds, a block of sulfur halide glass can be obtained. The precursor glass can be obtained by putting the sulfur halide glass into a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com