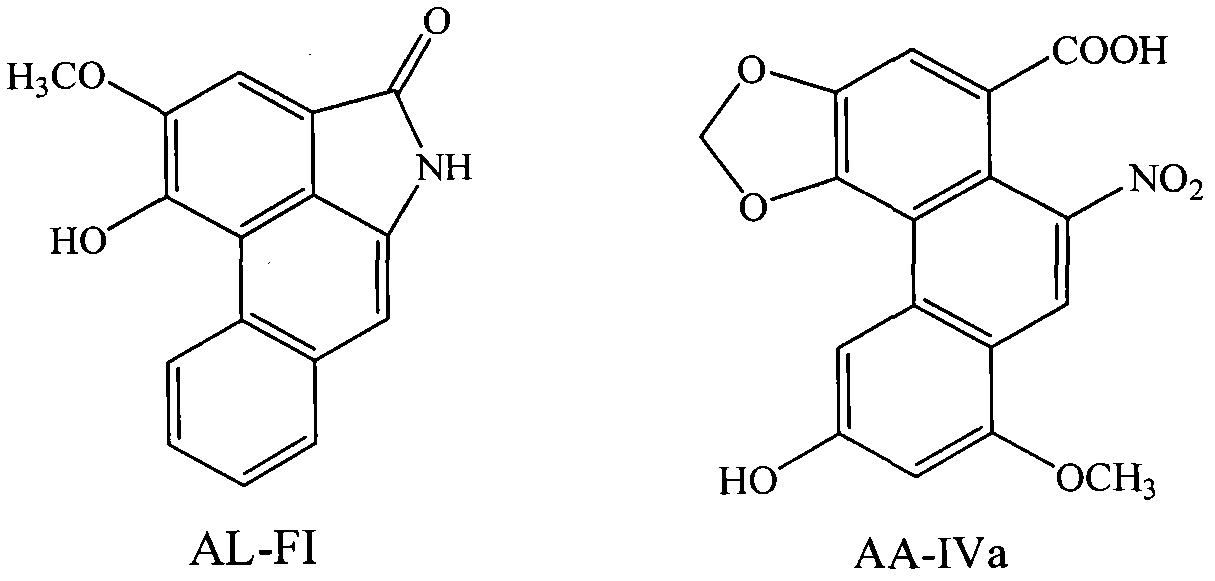
Preparation method of anti-aristololactam FI and aristolochic acid IVa monoclonal antibody, and application of monoclonal antibody
A technology of aristolochid lactam and monoclonal antibody, applied in chemical instruments and methods, anti-plant immunoglobulin, instruments, etc., can solve the problems of high requirements for operators and instrument maintenance, complicated sample pretreatment, and environmental protection question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
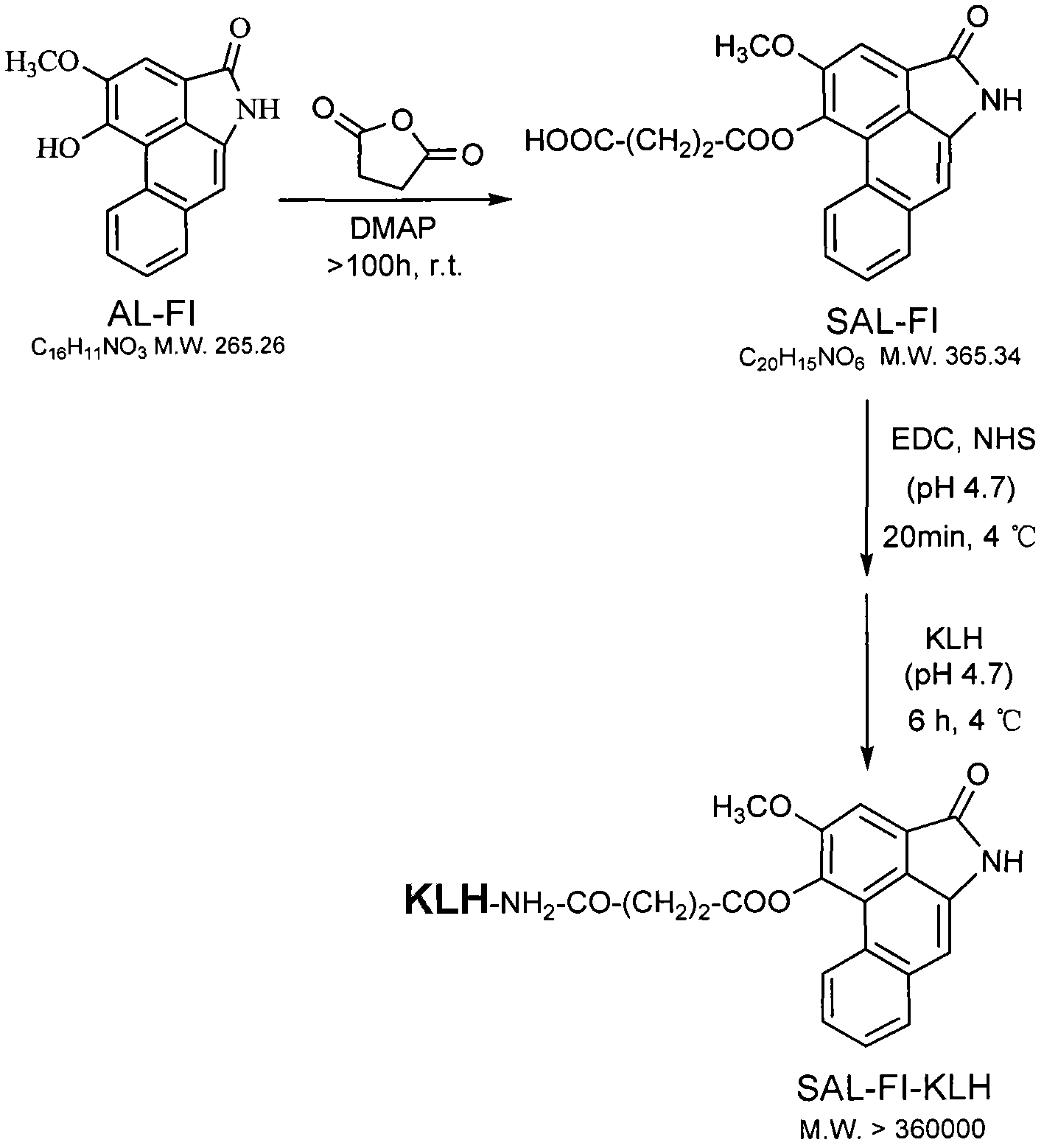
[0036] Synthesis of embodiment 1AL artificial hapten, immune antigen and coating antigen
[0037] 1. Synthesis of artificial hapten SAL-FI
[0038] Dissolve 70 mg of AL-FI (>98%) in 7 mL of pyridine, stir until completely dissolved, add 20 times the amount of succinic anhydride and 0.5 times the amount of catalyst 4-dimethylaminopyridine (DMAP), and stir magnetically at room temperature for 111 hours . After the reaction is completed, add 10% concentrated hydrochloric acid aqueous solution dropwise to the reaction liquid to adjust the pH to 3 to neutralize pyridine, then extract the reaction liquid several times with an equal volume of ethyl acetate, and finally wash the ethyl acetate layer with pure water several times to pH was 7, the ethyl acetate layers were combined and the solvent was recovered to obtain a crude product. Purify by preparative liquid chromatography to obtain the artificial hapten SAL-FI.
[0039] 2. Preparation of artificial immune antigen (SAL-FI-KLH)...
Embodiment 2
[0043] The preparation of embodiment 2 monoclonal antibody
[0044] 1. Animal immunity
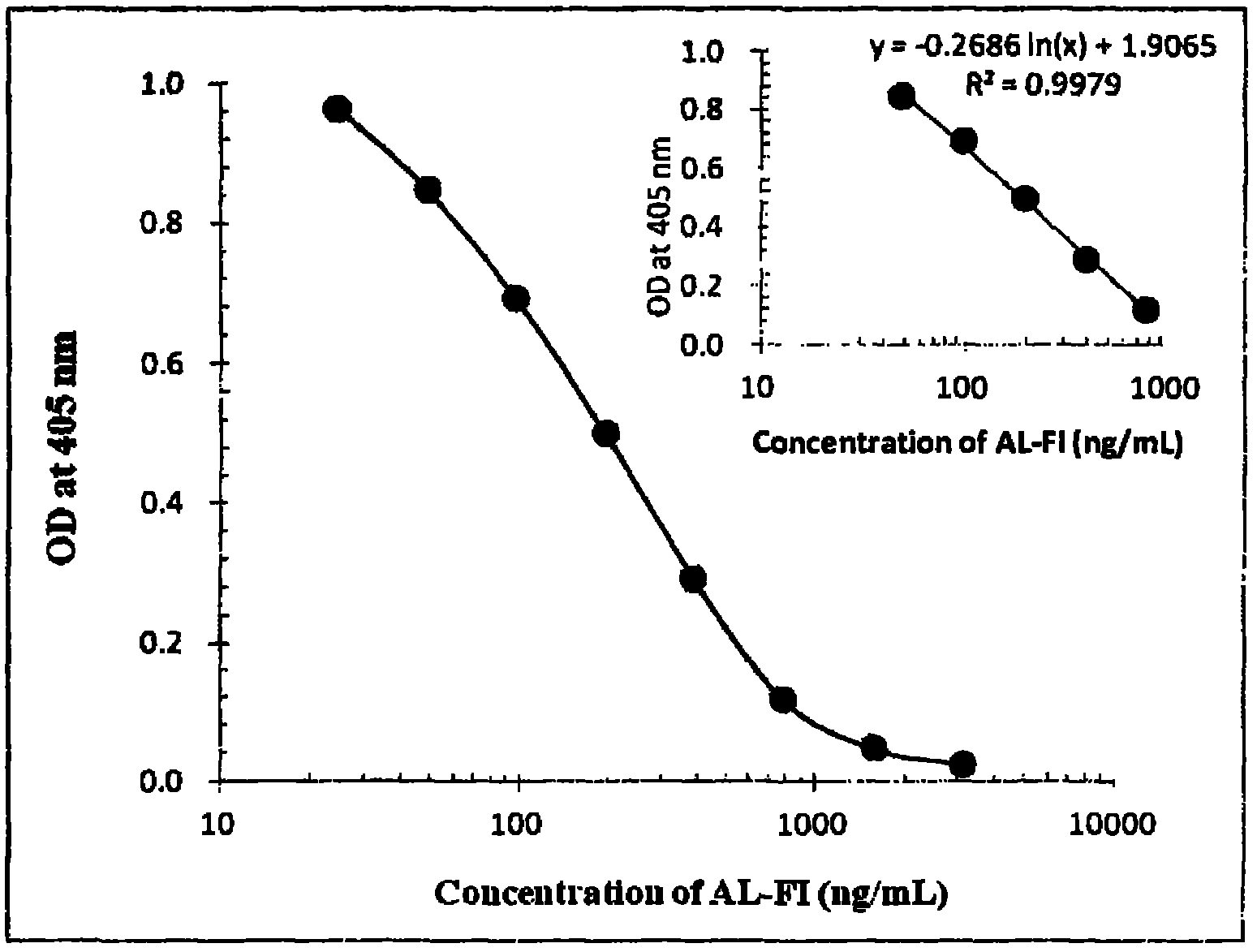
[0045] The 8M urea solution containing 5mg / mL SAL-FI-KLH was diluted 25 times with PBS, mixed with an equal volume of Freund's complete adjuvant to form an emulsion, and several 7-week-old male BALB / c mice were injected intraperitoneally, each 0.5 mL (50 μg). Two weeks after the first immunization, the same dose was used to boost the immunization, and the adjuvant was replaced by Freund's incomplete adjuvant. After the second immunization, the PBS solution of SAL-FI-KLH (without adjuvant) was injected intraperitoneally, 0.5 mL (100 μg) per mouse, and immunized twice with an interval of 2 weeks between each time. After the second and fourth immunizations, the titer of antiserum and the competitive inhibition rate of AL-FI were detected by ELISA method, and the one with better immune effect was selected, that is, the one with a serum titer of 1187 and 10 μg / mL AL-FI. Spleen cells (6×10 8...
Embodiment 3
[0059] Identification of embodiment 3 monoclonal antibody
[0060] 1. Monoclonal antibody purity test, antibody typing and potency (Ka) determination
[0061] The purity of the above-mentioned monoclonal antibody 1F9-9B-4G determined by the sandwich ELISA method was 33.38%; according to the instructions of the mouse antibody heavy chain isotype determination kit and the mouse antibody κ and λ chain rapid detection test strips, The purified monoclonal antibody solution was taken for operation, and the heavy chain type of the monoclonal antibody 1F9-9B-4G was determined to be IgG1, and the light chain type was κ. Referring to Friguet et al. [21] The antibody titer was detected by the method, and the affinity constant (Ka) of monoclonal antibody 1F9-9B-4G to AL-FI was 1.941×10 9 m -1 .
[0062] 2. Antibody specificity
[0063] Competitive ELISA was used to measure the specificity of the monoclonal antibody of the above invention and the cross-reactivity with similar compounds ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com