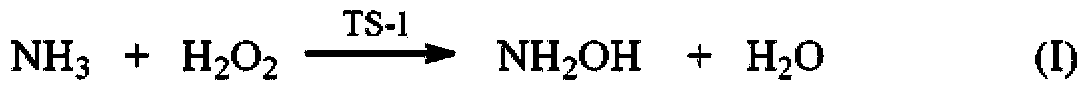Method for synthesizing hydroxylamine salt
A technology of hydroxylamine salt and cyclohexanone oxime, which is applied in the field of preparation of hydroxylamine salt, can solve the problems of low yield of hydroxylamine product, unindustrialization and the like, and achieves the effect of improving atom utilization rate and improving atom utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
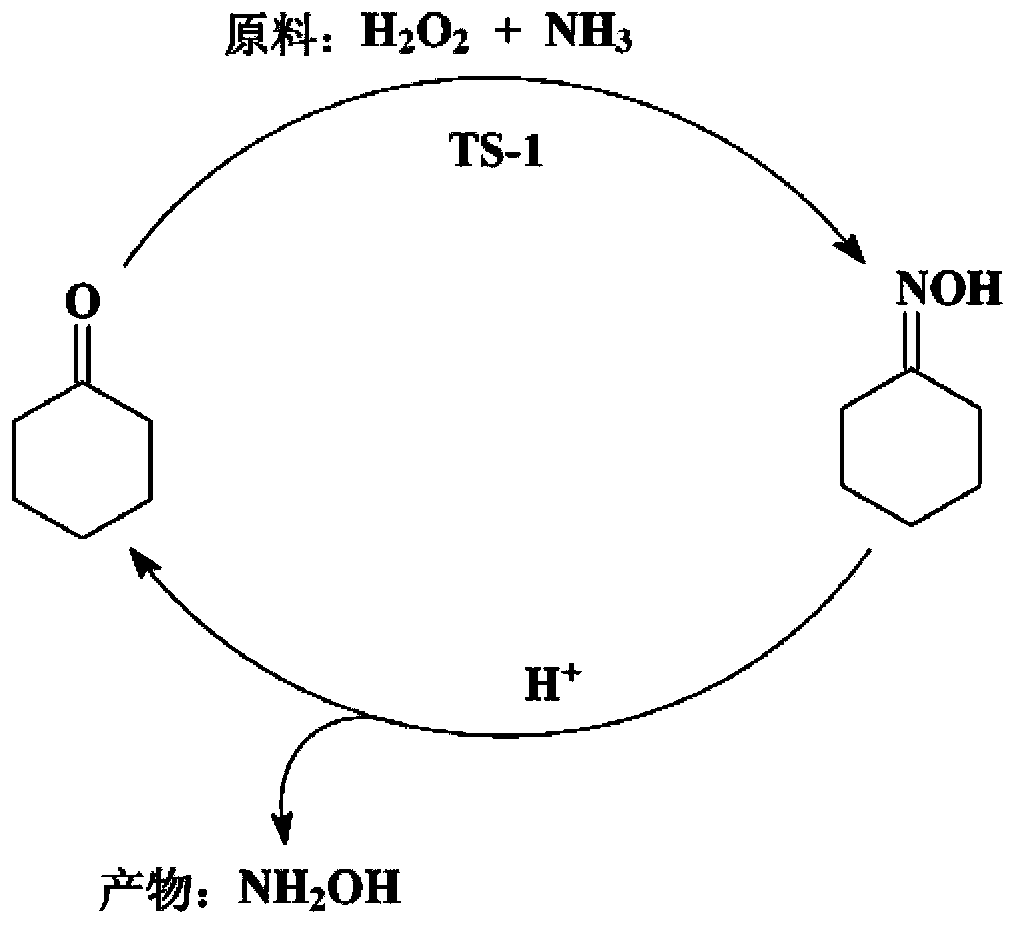
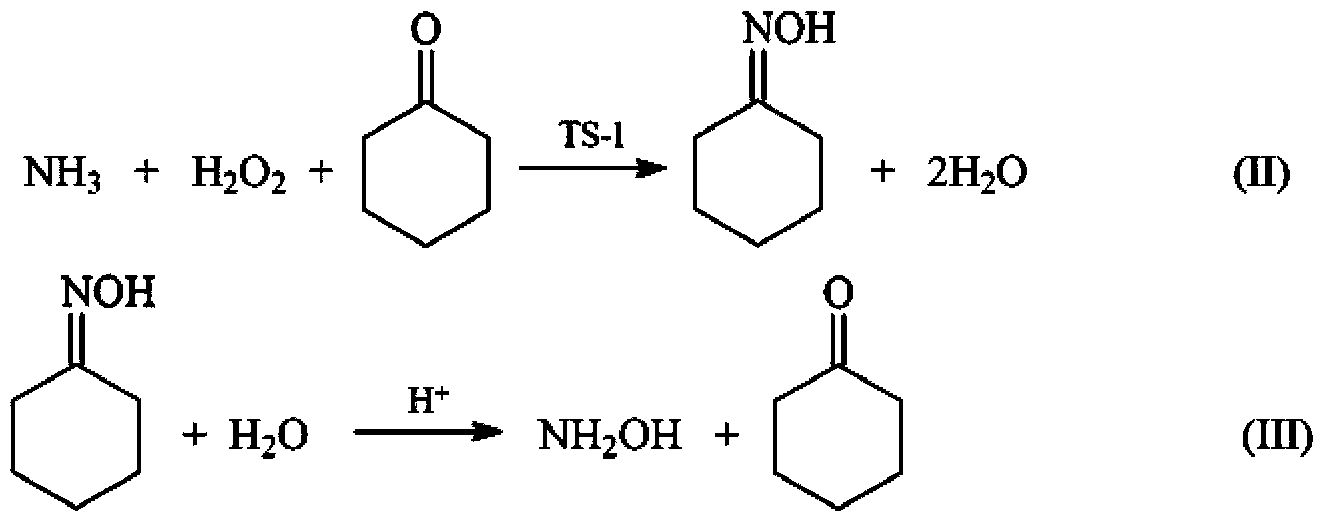
[0029] (1) Synthesis of cyclohexanone oxime reaction process: Weigh 0.6g of TS-1 molecular sieve and place it in the reactor, then measure 15ml (833.3mmol) of water, 6.4ml (61.6mmol) of cyclohexanone with a content of 99.5% and 25% 10ml of ammonia water (NH 3 The molar mass is 133.6mmol, H 2 O molar weight is 379.2mmol) is added in the reactor; Then, stir, heat up, and reaction liquid is warmed up to 70 ℃ by room temperature, dropwise adds 30% hydrogen peroxide 8ml (H 2 o 2 The molar mass is 79.7mmol, H 2 O molar weight is 351.6mmol), normal pressure reaction 0.25h, reaction finishes. The reaction solution and solid TS-1 catalyst were separated by centrifugation. Toluene was used as the extractant to extract and separate the organic phase in the reaction solution. The content of cyclohexanone oxime in the organic phase was analyzed by gas chromatography. The yield of the intermediate product cyclohexanone oxime was 99.2%. At the same time, the TS-1 catalyst obtained by ce...
Embodiment 2~3
[0034] Same as the operating steps and reaction conditions of the process of preparing hydroxylamine by hydrolysis of cyclohexanone oxime in implementation 1, except that the inorganic acid added in the hydrolysis process is respectively 2ml of concentrated sulfuric acid (H + The molar mass is 71.3mmol, H 2 O molar weight is 10.2mmol), 65% concentrated nitric acid 5ml (H + The molar mass is 72.5mmol, H 2 O molar weight is 136.6mmol), and the finally obtained raffinate aqueous phase is respectively hydroxylamine sulfate mother liquor, hydroxylamine nitrate mother liquor. The organic phase was analyzed by gas chromatography, and the yield of hydroxylamine was quantitatively calculated. The experimental results are shown in Table 2.
[0035] Table 2 Influence of the type of inorganic acid on the hydrolysis reaction of cyclohexanone oxime to produce hydroxylamine
[0036] Example
Embodiment 4
[0038] The operation steps and reaction conditions are the same as in Example 1, except that in the process of producing hydroxylamine by hydrolysis of cyclohexanone oxime, the addition of concentrated hydrochloric acid is changed to 0.5ml (H + The molar mass is 5.9mmol, H 2 O molar weight is 21.3mmol). The organic phase was analyzed by gas chromatography, and the yield of cyclohexanone oxime in the reaction process (1) and the yield of hydroxylamine in the reaction process (2) were quantitatively calculated. The experimental results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com