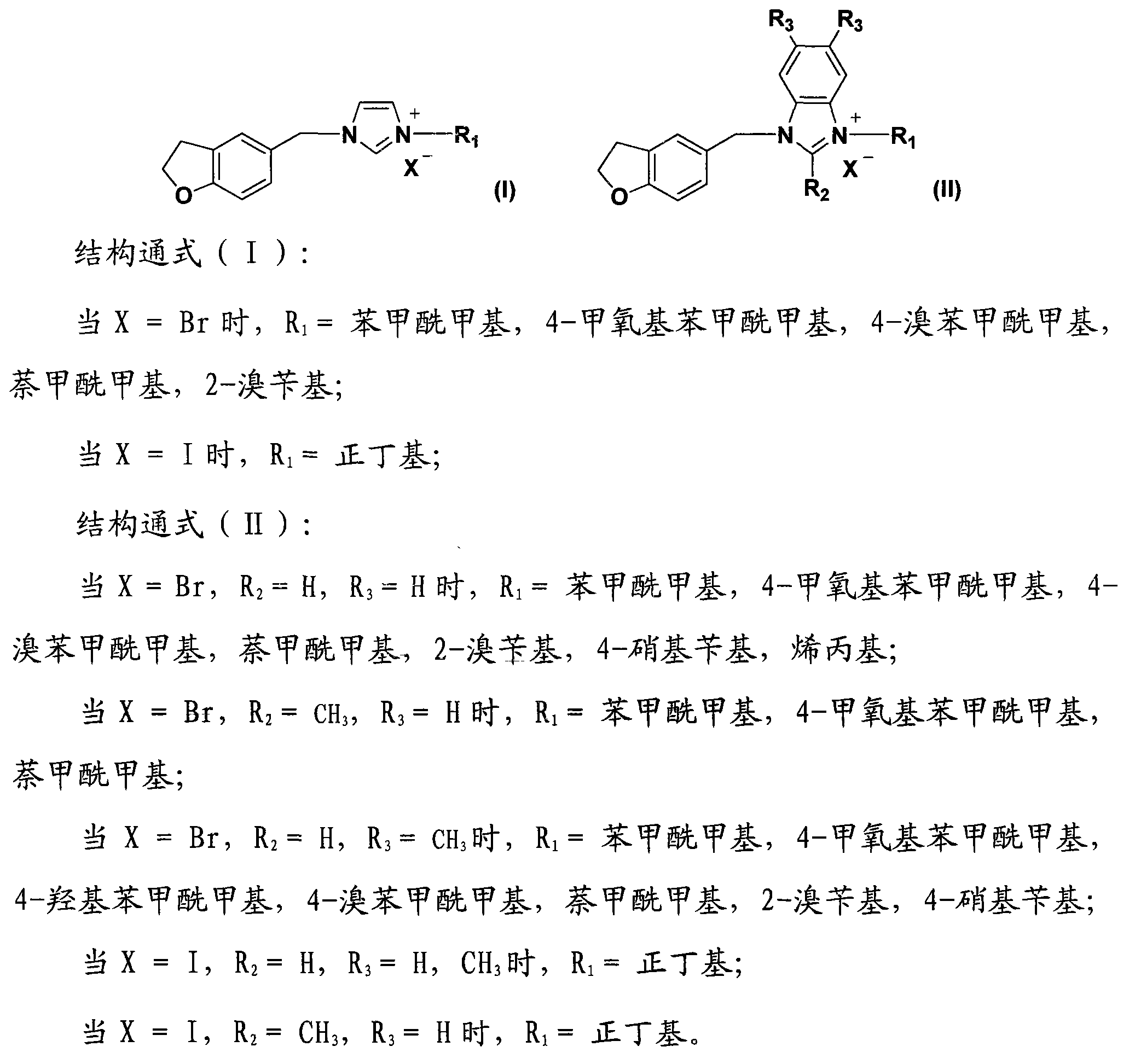
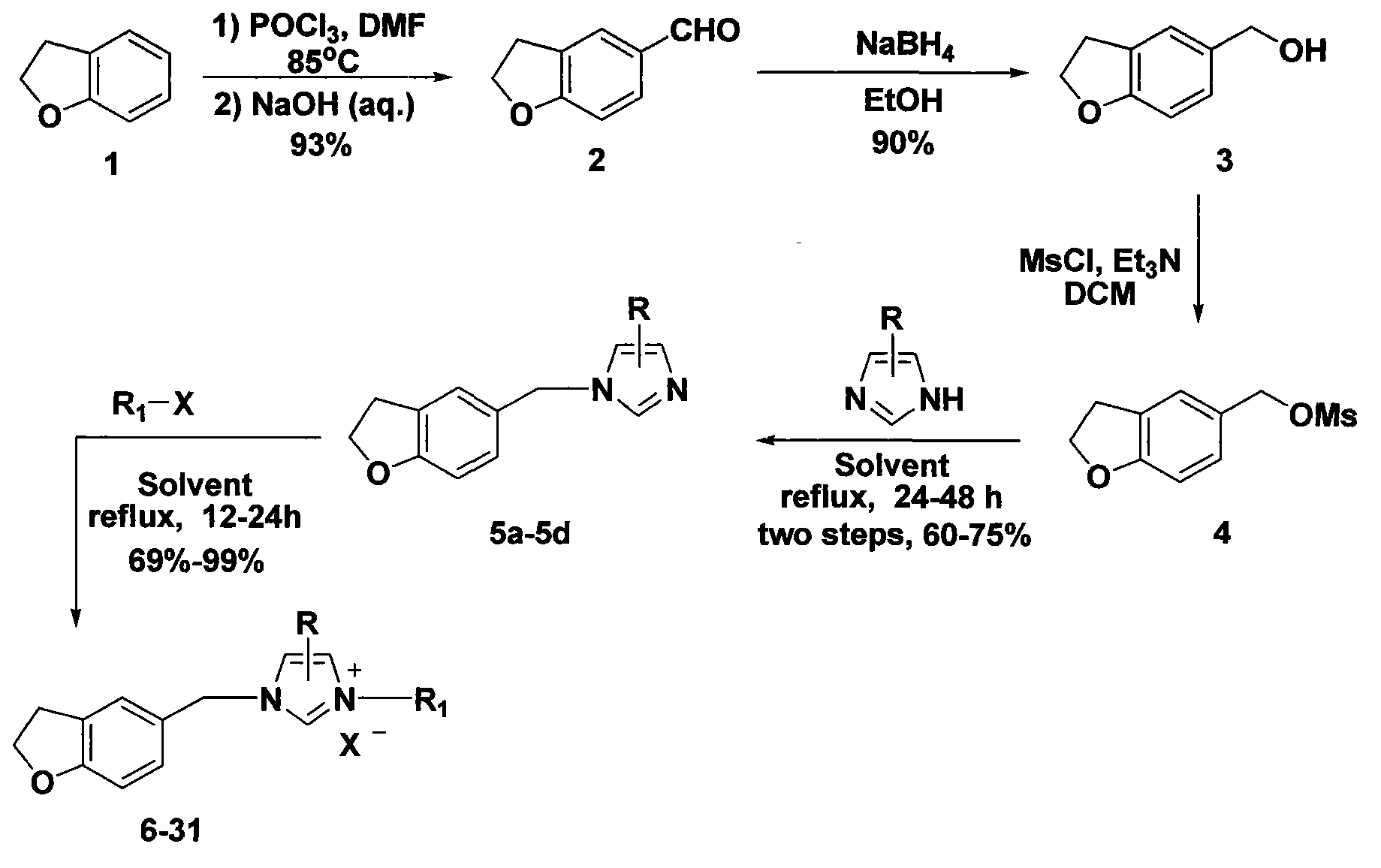
5-substituted dihydrobenzofuran-imidazolium salt compound and preparation method thereof
The technology of a salt compound and benzimidazole is applied in the application field of anti-cancer, which can solve the problems of lack of manufacturing methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 11-(5-Methyl-substituted dihydrobenzofuran)-3-(phenacylmethyl)imidazolium bromide
[0041]
[0042] The preparation process is as follows:
[0043] 1. Preparation of 5-formyl dihydrobenzofuran (2):
[0044] Under ice-water bath, slowly dropwise add phosphorus oxychloride (31.0ml, 0.333mol) into N,N-dimethylformamide (28.4ml, 0.366mol), react for 10 minutes, slowly dropwise add 2,3-di Hydrobenzofuran (1, 20.0g, 0.166mol), remove the ice-water bath, heat and stir at 85°C for 12 hours, pour the reaction system into ice water, add sodium hydroxide solution, adjust the pH value to 8-9, and use acetic acid Ethyl ether extraction, the organic phase washed with saturated brine, anhydrous Na 2 SO 4 After drying, filtering, and concentrating the solvent under reduced pressure, silica gel column chromatography (100-200 mesh) with petroleum ether-ethyl acetate (5:1) as the eluent was used to prepare 5-formyl dihydrobenzofuran ( 2, 22.8g), yield 93%;
[0045] The pre...
Embodiment 2
[0056] Example 21-(5-Methyl-substituted dihydrobenzofuran)-3-(4-methoxyphenacylmethyl)imidazolium bromide
[0057]
[0058] The preparation process is as follows:
[0059] 1. Preparation of 5-formyl dihydrobenzofuran (2): The method is the same as in Example 1.
[0060] 2. Preparation of 5-benzyl alcohol substituted dihydrobenzofuran (3): The method is the same as in Example 1.
[0061] 3. Preparation of 1-(5-methyl-substituted dihydrobenzofuran)imidazole (5a): The method is the same as in Example 1.
[0062] 4. Preparation of 1-(5-methyl-substituted dihydrobenzofuran)-3-(4-methoxyphenacyl)imidazolium bromide (7):
[0063] Dissolve 1-(5-methyl-substituted dihydrobenzofuran) imidazole (5a, 200mg, 1mmol) in toluene (20ml), add 4-methoxyphenacyl bromide (274mg, 1.2mmol) under stirring , the reaction was stirred and refluxed for 20 hours, cooled to room temperature, a solid precipitated out, filtered, the precipitate was washed several times with acetone, and dried to prepar...
Embodiment 3
[0069] Example 31-(5-Methyl-substituted dihydrobenzofuran)-3-(4-bromophenacylmethyl)imidazolium bromide salt
[0070]
[0071] The preparation process is as follows:
[0072] 1. Preparation of 5-formyl dihydrobenzofuran (2): The method is the same as in Example 1.
[0073] 2. Preparation of 5-benzyl alcohol substituted dihydrobenzofuran (3): The method is the same as in Example 1.
[0074] 3. Preparation of 1-(5-methyl-substituted dihydrobenzofuran)imidazole (5a): The method is the same as in Example 1.
[0075] 4. Preparation of 1-(5-methyl-substituted dihydrobenzofuran)-3-(4-bromophenacyl)imidazolium bromide (8):
[0076] 1-(5-Methyl-substituted dihydrobenzofuran) imidazole (5a, 200mg, 1mmol) was dissolved in toluene (20ml), and 4-bromophenacyl bromide (331mg, 1.2mmol) was added under stirring, and the reaction Stirred and refluxed for 24 hours, cooled to room temperature, a solid precipitated out, filtered, the precipitate was washed several times with acetone, dried,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com