Synthesis and applications of trapezoid condensed polycyclic conjugation semiconductor molecules and polymers
A polymer, fused polycyclic technology, applied in the direction of semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problems that have not been used to synthesize polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
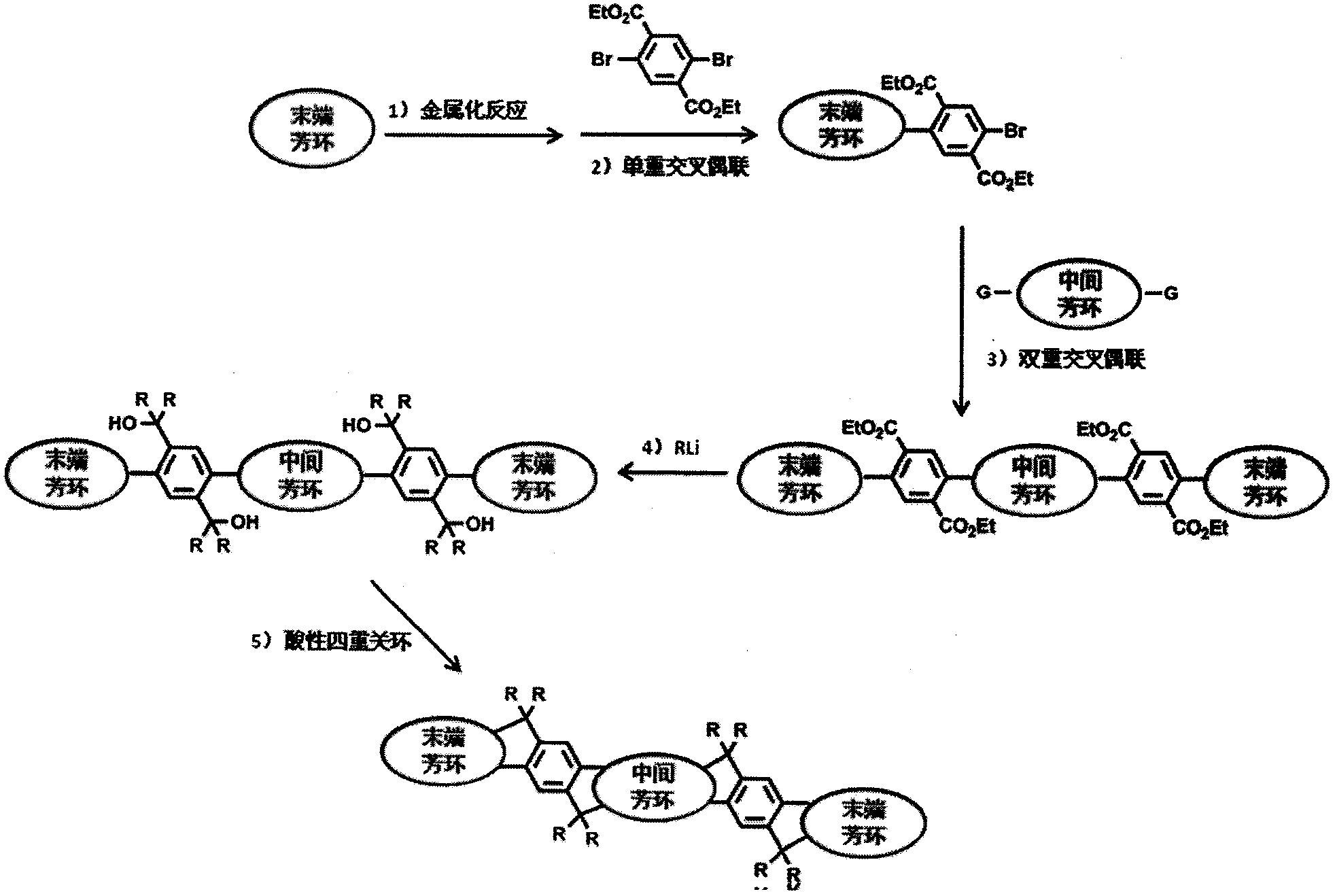
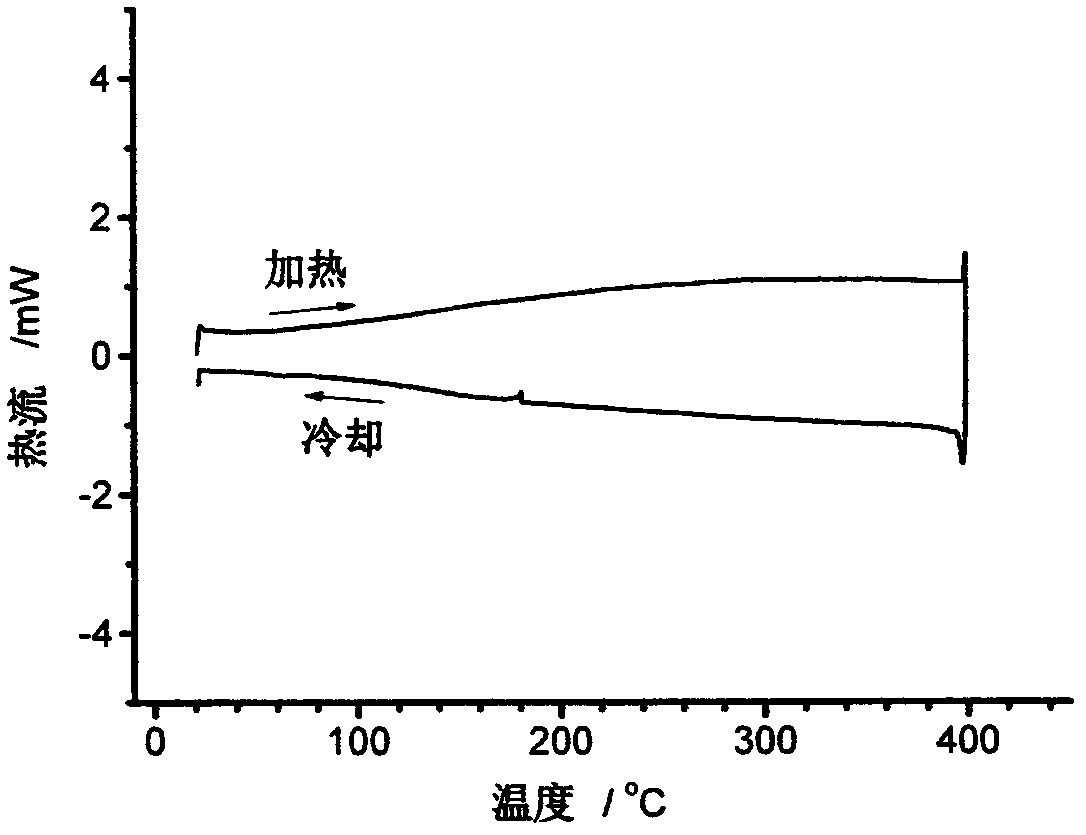
Image
Examples
Embodiment 1
[0058] Synthesis of dihexyl 2-bromo-5-thieno[3,2-b]thienyl terephthalate (Intermediate 4):
[0059] The specific steps are:
[0060] In a solution of thieno[3,2-b]thiophene (3.51g, 25mmol) in anhydrous tetrahydrofuran (50ml) at -70°C, add 2.5M n-butyllithium hexane solution (10.5ml, 26.5mmol) dropwise with stirring , the dropping time is 15 minutes. Stirring of the resulting mixture was continued for 1 hour with cooling. Tributyltin chloride (7.42ml, 26.5mmol) was added in one go with a syringe, and the resulting yellow solution was stirred at low temperature for 10 minutes. The cooling bath was removed, and the reaction was continued at room temperature for 1 hour. The solvent was removed from the obtained mixture with a rotary evaporator, and the remaining liquid was dissolved in petroleum ether. The solution was filtered with a microporous membrane filter, and the filtrate was spin-dried in vacuum. The remaining oily liquid was dissolved in toluene (30ml), and 2,5-dibr...
Embodiment 2
[0062] Synthesis of intermediate 5
[0063] The specific steps are:
[0064] According to the synthesis method similar to intermediate 4, n-butyllithium was added dropwise to a solution of thieno[3,2-b]thiophene (0.673g, 4.8mmol) in anhydrous tetrahydrofuran (20ml) at -70°C with stirring Hexane solution (2.5M, 3.90ml, 9.75mmol), the dropwise addition time was 5 minutes. Stirring of the resulting mixture was continued for 1 hour with cooling. Tributyltin chloride (2.65ml, 9.77mmol) was added in one portion with a syringe, and the resulting yellow solution was stirred at low temperature for 10 minutes. The cooling bath was removed, and the reaction was continued at room temperature for 1 hour. The solvent was removed from the obtained mixture with a rotary evaporator, and the remaining liquid was dissolved in petroleum ether. The solution was filtered with a microporous membrane filter, and the filtrate was spin-dried in vacuum. The remaining oily liquid (this is Intermedia...
Embodiment 3
[0066] Synthesis of compound 7 (R=4-n-hexadecylphenyl)
[0067] The specific steps are:
[0068] First, a solution of 4-n-hexadecyl-1-bromobenzene (9.76g, 25mmol) in anhydrous tetrahydrofuran (150ml) was cooled to -25°C, and then 2.5M n-butylene was added dropwise thereto within 30 minutes under stirring Lithium hexane solution (10ml, 25mmol). The obtained slightly yellow emulsion mixture was stirred at the same temperature for 1 hour, and then compound 5 (2.14 g, 2.5 mmol) was added as a solid. The mixture was first cooled and stirred for 4 hours, and then stirred at room temperature for 4 hours until all the solids were dissolved. Distilled water (50 ml) was added to the reaction flask, followed by vigorous stirring at room temperature for 20 minutes. The mixture was transferred into a separatory funnel, the upper orange organic phase was separated, the lower aqueous phase was extracted with ether (50ml), the organic phases were combined, dried over anhydrous magnesium su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com