High-level soluble expression method and application of recombined tyrosine decarboxylase
A technology of tyrosine decarboxylase and recombinant plasmid, which is applied in the field of enzyme engineering, can solve the problems of a large number of inclusion bodies, and achieve the effects of high expression level, high recovery rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Cloning and preliminary expression of Lactobacillus breve tyrosine decarboxylase TDC gene
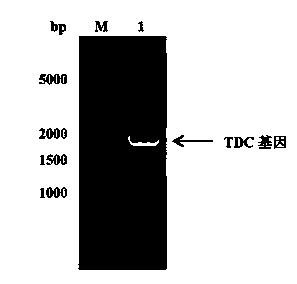
[0080] The TDC gene used in the present invention is derived from Lactobacillus brevis Lactobacillus brevis CGMCC 1.2028, the primer design refers to the gene sequence encoding TDC in GenBank (EU195891.1), the gene length is 1881 bp, encoding 626 amino acids. The upstream primer is shown in SEQ ID No. 2, adding Nhe I enzyme cutting site; Downstream primer is shown in SEQ ID No. 3, adds in 5 ' end xho I restriction site. The TDC gene fragment was obtained by PCR amplification, and the reaction system was as follows:
[0081]
[0082] PCR reaction program: denaturation at 94°C for 10 min, denaturation at 94°C for 1 min, annealing at 57°C for 45 s, extension at 72°C for 2 min, after 30 cycles of reaction, extension at 72°C for 10 min, and finally cooling to 4°C. After PCR, 5 μL of the product was used for agarose gel electrophoresis, the agarose concentration...
Embodiment 2
[0105] Embodiment 2: the optimization of the fermentation medium of TDC recombinant bacterium and fermentation enzyme production condition
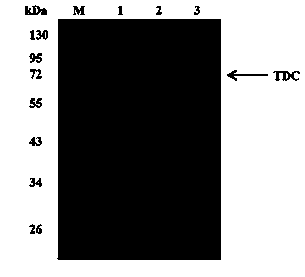
[0106] The TDC recombinant bacteria were cultured in a 250 mL shake flask containing 30 mL LB / Kan medium for 12 h, and inoculated into LBG / Kan medium at an inoculum size of 2% to induce rTDC expression. Figure 4 The expression of rTDC within 6 h of induction of the recombinant bacteria. It can be seen that with the prolongation of the induction time, both the biomass and the yield of rTDC showed an increasing trend, and the expression of protein was greatly increased compared with that before optimization. Figure 4 The fermentation conditions are: OD after transfer 600 The induction started when it reached 0.5, the IPTG concentration was 0.4 mM, the induction temperature was 25°C, and 180 rpm.
[0107] After optimizing the fermentation medium, there was almost no inclusion body formation within the first 6 hours of rTDC induction. Af...
Embodiment 3
[0115] Example 3: Purification and enzymatic property determination of recombinant tyrosine decarboxylase
[0116] All purification operations were performed at or near 4°C at low temperature.
[0117] The induced recombinant bacterial fermentation broth was centrifuged at 8000 rpm for 10 min to collect the bacterial cells. The pellet was washed twice with buffer A and then resuspended. The cells were disrupted by ultrasonication or high-pressure homogenization, and centrifuged again. The obtained supernatant was rTDC crude enzyme solution.
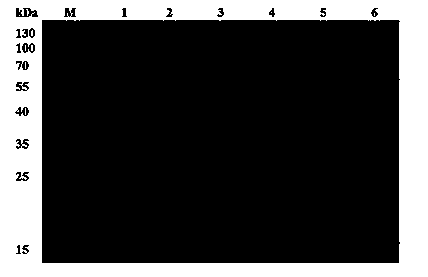
[0118] First equilibrate the Ni column with buffer A at a flow rate of 2 mL / min for 10 min, then load the sample at a flow rate of 2 mL / min, continue to wash with buffer A to the baseline after loading the sample, and then replace buffer B to elute rTDC , the eluent flow rate is 2 mL / min, the elution process is as follows Figure 5 shown. The purity of rTDC after purification by Ni column and the size of rTDC single subunit are as foll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene length | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com