Manganese chloride and preparation method thereof
A technology of manganese chloride and ammonium chloride solution, which is applied to manganese halides, electrochemical generators, electrical components, etc., and can solve the problems of high power consumption in production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
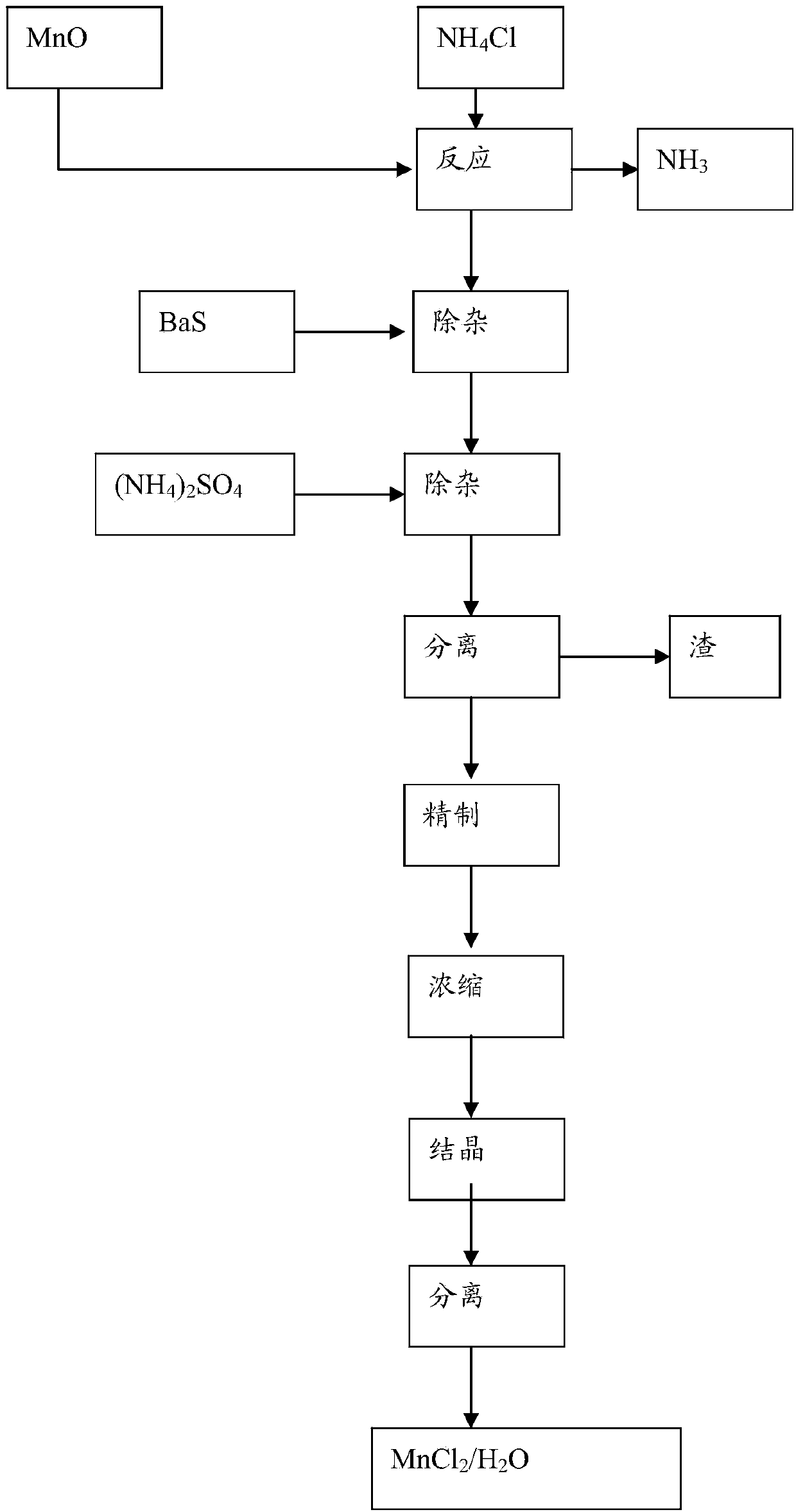
[0026] A preparation method of manganese chloride, the method comprising:
[0027] (1) Metathesis reaction
[0028] According to Mn 2+ with NH 4+ The molar ratio of the manganese oxide is 1:2.4~2.8, mix the manganous oxide and the ammonium chloride solution, and then heat the reaction at 85-90°C for 2-3 hours.
[0029] The chemical reaction equation involved in this step is: NH produced by absorption 3 The gas is used in the production of high-purity barium carbonate.
[0030] This manganous oxide can be conventional commercially available manganous oxide, and also can be the manganous oxide that barium manganese combines technology to obtain, specifically will [Ba 2+ ] is 0.70~1.5mol / L BaS solution molar ratio is manganese:sulfur=1:1~1.1 Add manganese oxide ore powder to react, control the temperature of the reaction system in the range of 60~90°C, and the reaction time is controlled at 1~ After 2.5 hours, solid-liquid separation was carried out after the reaction, and...
Embodiment 1
[0049] (1) Metathesis reaction
[0050] According to Mn 2+ with NH 4+ The molar ratio of the manganese oxide is 1:2.4, mix the manganous oxide and the ammonium chloride solution, and then heat the reaction at 85°C for 2 hours.
[0051] (2) Impurity removal
[0052] According to [Fe 2+ ] / [S 2- ]=1 / 1.05 molar ratio, add 100g / L barium sulfide solution to the reaction solution after heating reaction in step (1), and stir for 60 minutes.
[0053] According to [Ba 2+ ] / [SO 4 2- ] equal molar ratio, add industrial ammonium sulfate in the reaction solution, then continue to add ammonium sulfate to make [SO 4 2- ]≥0.05mol / L, stirred and reacted for 30 minutes, separated by pressure filtration, discarded after washing the filter residue, and the obtained filtrate was sent to the next step.
[0054] (3) Refining, evaporation, crystallization
[0055] Acidify the filtrate obtained in step (2) to pH 4 with industrial hydrochloric acid, add hydrogen peroxide and heat to boil for ...
Embodiment 2
[0059] (1) Metathesis reaction
[0060] According to Mn 2+ with NH 4+ The molar ratio is 1:2.6, manganous oxide and ammonium chloride solution are mixed, and then heated at 87°C for 2.5 hours.
[0061] (2) Impurity removal
[0062] According to [Fe 2+ ] / [S 2- ]=1 / 1.2 molar ratio, add 120g / L barium sulfide solution to the reaction solution after heating reaction in step (1), and stir for 75 minutes.
[0063] According to [Ba 2+ ] / [SO 4 2- ] equal molar ratio, add industrial ammonium sulfate in the reaction solution, then continue to add ammonium sulfate to make [SO 4 2- ]≥0.05mol / L, stirred and reacted for 45 minutes, separated by pressure filtration, discarded after washing the filter residue, and the obtained filtrate was sent to the next step.
[0064] (3) Refining, evaporation, crystallization
[0065] Acidify the filtrate obtained in step (2) to pH 3.5 with industrial hydrochloric acid, add hydrogen peroxide and heat to boil for 45 minutes, then NH 3 Adjust the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com