Synthesis method of 1,2,4-trifluoro benzene
A synthesis method and technology of trifluorobenzene, applied in chemical instruments and methods, halogenated hydrocarbon preparation, organic chemistry and other directions, can solve the problems of unsatisfactory product purity, harsh reaction conditions, high price, etc., and achieve less impurities and operating conditions. The effect of stable and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
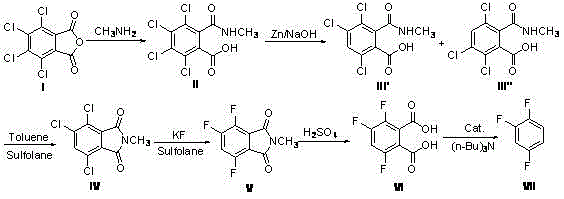
[0064] Add 166 grams of 30% methylamine aqueous solution into a 500 ml reaction bottle, stir at room temperature, add 114.4 grams of tetrachlorophthalic anhydride (I) in batches, and after the addition is completed, heat up to 40-50o C was reacted for 3 hours and cooled to room temperature to obtain compound (II).
[0065] To the above-mentioned system containing compound (II), add 240 grams of 20% sodium hydroxide solution, stir and cool to -10~0 o C, add 65 grams of zinc powder, temperature control -10 ~ 0 o C reacted for 20 hours, removed the low-temperature bath, returned to room temperature, filtered, and washed the filter cake with water. The filtrate and the washing liquid were combined, the pH was adjusted to acidic with concentrated hydrochloric acid, filtered, and the filter cake was drained to obtain a mixture of compound (III') and compound (III"), with a wet weight of 102 grams.
[0066] In a 500 ml reaction flask, add the mixture of the above compound (III...
Embodiment 2
[0071] Add 155 grams of 40% methylamine aqueous solution in 1 liter reaction bottle, stir and cool to 10 o C, add 286 grams of tetrachlorophthalic anhydride (I) in batches, after adding, control the temperature for 10-20 o C was reacted for 6 hours to obtain compound (II).
[0072] To the above system containing compound (II), add 530 g of 15% sodium hydroxide solution, stir at room temperature, add 130 g of zinc powder, react at room temperature for 10 hours, filter, and wash the filter cake with water beating. Combine the filtrate and the washing liquid, adjust the pH to acidic with concentrated hydrochloric acid, filter, and drain the filter cake to obtain a mixture of compound (III') and compound (III"), with a wet weight of 260 grams.
[0073] In a 1-liter reaction flask, add the mixture of the above-mentioned compound (III') and compound (III"), add 130 grams of toluene, 650 grams of sulfolane, reflux for 15 hours, distill the toluene off, and cool down to room temp...
Embodiment 3
[0078] Add 186 grams of 35% methylamine aqueous solution into a 1-liter reaction bottle, stir at room temperature, add 200 grams of tetrachlorophthalic anhydride (I) in batches, and react at room temperature for 5 hours to obtain compound (II).
[0079] To the above-mentioned system containing compound (II), add 350 grams of 12% sodium hydroxide solution, and control the temperature for 10 to 20 o C, add 68.5 grams of zinc powder, after adding, in 10 ~ 20 o C was reacted for 15 hours. Filter, wash the filter cake twice with 50 g of 1% sodium hydroxide solution, combine the filtrate and washing liquid, adjust the pH to acidic with concentrated hydrochloric acid, filter, and drain the filter cake to obtain compound (III') and compound (III" ) with a wet weight of 168 grams.
[0080] In a 1-liter reaction flask, add the mixture of the above-mentioned compound (III') and compound (III"), add 380 grams of toluene, 420 grams of sulfolane, reflux for 18 hours, distill out the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com