Synthetic method of 1-cyclopropyl-4-oxo-7-fluoro-8-methoxy-1,4-dihydroquinoline-3-carboxylic acid
A dihydroquinoline and cyclopropyl technology, which is applied in the field of synthesis of new quinolone drug intermediates, can solve the problems of small supply, stable supply of raw materials, unsatisfactory atom economy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
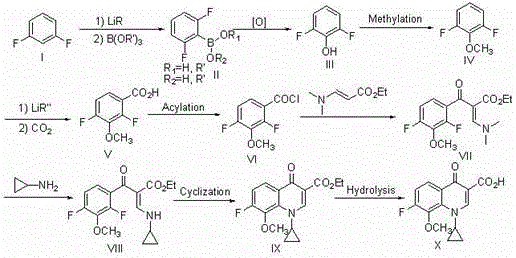
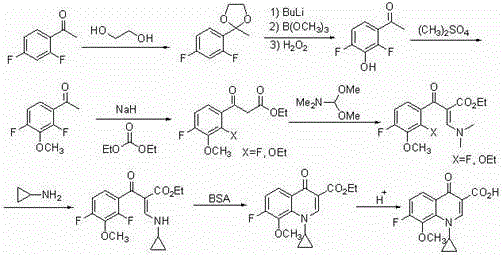
[0063] In a 500ml dry reaction flask, replace the gas with nitrogen, add 22.8g of compound (I) and 150ml of anhydrous ether, and stir and cool the system to -78°C under the protection of nitrogen. o C. Slowly add 187 ml of 1.6M n-butyllithium n-hexane solution dropwise. o C stirred the reaction for 2 hours. Cool the system back to -78 o C, add 60 grams of trimethyl borate dropwise, after the dropwise addition, control the temperature at -50~-60 o C stirred the reaction for 2 hours. Return the reaction system to room temperature, add 200 ml of water to quench the reaction, and adjust it to acidity with 36% hydrochloric acid. After heating up and distilling to recover the organic solvent, control the temperature at 70-80 o C stirred the reaction for 6 hours. The system was cooled and crystallized, filtered and washed with water to obtain 27.0 g of compound (II).
Embodiment 2
[0065] Add 31.6 grams of compound (II), 150 milliliters of ethanol, 100 milliliters of water, and 30 milliliters of acetic acid into a 500 milliliter reaction bottle, stir at room temperature, add 34 grams of 30% hydrogen peroxide dropwise, after the addition is complete, control the temperature for 20 o C stirred and reacted for 30 hours, the system was lowered to room temperature, 200 ml of 10% sodium bisulfite solution was added dropwise, stirred for 1 hour after the dropwise addition, ethanol was recovered by distillation under reduced pressure, and the residue was extracted 3 times with ethyl acetate, each dosage 100 ml, the organic phases were combined, dried and concentrated to obtain 23.9 g of compound (III).
Embodiment 3
[0067] Add 26.2 grams of compound (III), 200 milliliters of tetrahydrofuran, and 83 grams of potassium carbonate to a 500 milliliter reaction bottle, stir at room temperature, add 38 grams of dimethyl sulfate dropwise, after the addition is complete, continue to stir for 10 hours, filter, and depressurize the filtrate Concentrate, recover the solvent, and distill the residue to obtain 26.8 g of compound (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com