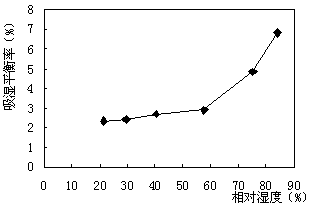
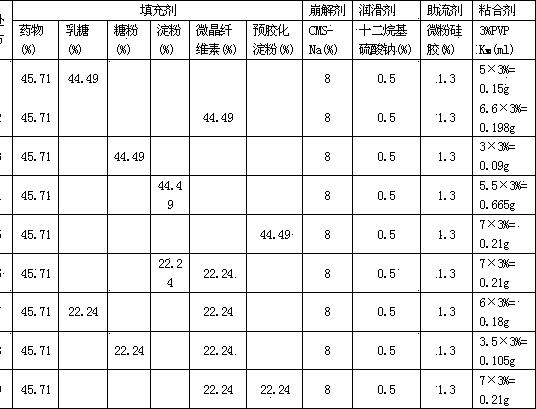
Medicament for treating dysmenorrhea and preparation method thereof
A technology for drugs and dysmenorrhea, which can be used in pharmaceutical formulations, drug combinations, drug delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Prescription: 160g total flavonoid aglycone of Scutellaria baicalensis, 84g microcrystalline cellulose, 62.48g pregelatinized starch, 21g sodium carboxymethyl starch, 7g crospovidone, 1.75g sodium lauryl sulfate, polysorbate 80 1.22g, talcum powder 4.55g, povidone K 30 8g.
[0143] Process: the dispersible tablet is prepared like this:
[0144] A, take by weighing sodium lauryl sulfate, polysorbate 80 and povidone K 30 Formulated with Povidone K 30 It is a 5% aqueous solution, which is product A;
[0145] B, take by weighing the Scutellaria baicalensis total flavonoid aglycon of 100 mesh sieves, add microcrystalline cellulose, pregelatinized starch and sodium carboxymethyl starch, mix homogeneously in the mixing granulator, get B product;
[0146] C. Mix product A and product B, add 0.06 times the amount of distilled water of product B, stir, granulate, and obtain granules, which is product C;
[0147] D. Take product C and dry it at 70°C for...
Embodiment 2
[0150] Prescription: 170g total flavonoid aglycone of Scutellaria baicalensis, 90g microcrystalline cellulose, 70g pregelatinized starch, 25g sodium carboxymethyl starch, 10g crospovidone, 5g sodium lauryl sulfate, 5g polysorbate 80, Talc powder 5g, Povidone K 30 10g.
[0151] Process: the dispersible tablet is prepared like this:
[0152] A, take by weighing sodium lauryl sulfate, polysorbate 80 and povidone K 30 Formulated with Povidone K 30 It is a 5% aqueous solution, which is product A;
[0153] B, take by weighing the Scutellaria baicalensis total flavonoid aglycon of 120 mesh sieves, add microcrystalline cellulose, pregelatinized starch and sodium carboxymethyl starch, mix homogeneously in the mixing granulator, get B product;
[0154] C. Mix product A and product B, add 1 times the amount of water of product B, stir, granulate, and obtain granules, which is product C;
[0155] D. Take product C and dry it at 80°C for 4 hours. After the dried g...
Embodiment 3
[0158] Prescription: 150g total flavonoid aglycone of Scutellaria baicalensis, 80g microcrystalline cellulose, 60g pregelatinized starch, 15g sodium carboxymethyl starch, 1g crospovidone, 0.1g sodium lauryl sulfate, 0.5 polysorbate 80 g, talcum powder 3g, povidone K 30 1g.
[0159] Process: the dispersible tablet is prepared like this:
[0160] A, take by weighing sodium lauryl sulfate, polysorbate 80 and povidone K 30 Formulated with Povidone K 30 It is a 5% aqueous solution, which is product A;
[0161] B, take by weighing the Scutellaria baicalensis total flavonoid aglycon of 80 mesh sieves, add microcrystalline cellulose, pregelatinized starch and sodium carboxymethyl starch, mix homogeneously in the mixing granulator, get B product;
[0162] C. Mix product A and product B, add 0.03 times the amount of water of product B, stir, granulate, and obtain granules, which is product C;
[0163] D. Take product C and dry it at 60°C for 2 hours. After the drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com