Epitope antigens of human infection with H7N9 avian influenza and applications of epitope antigens in immune detection reagent
A technology for immune detection and avian influenza, which is applied in the field of detection of antibodies against human infection with H7N9 avian influenza. It can solve the problems of unsound sentences and affecting the specificity of H7N9 immune detection, and achieve good repeatability, stable detection results and high application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The selection of embodiment 1 candidate epitope
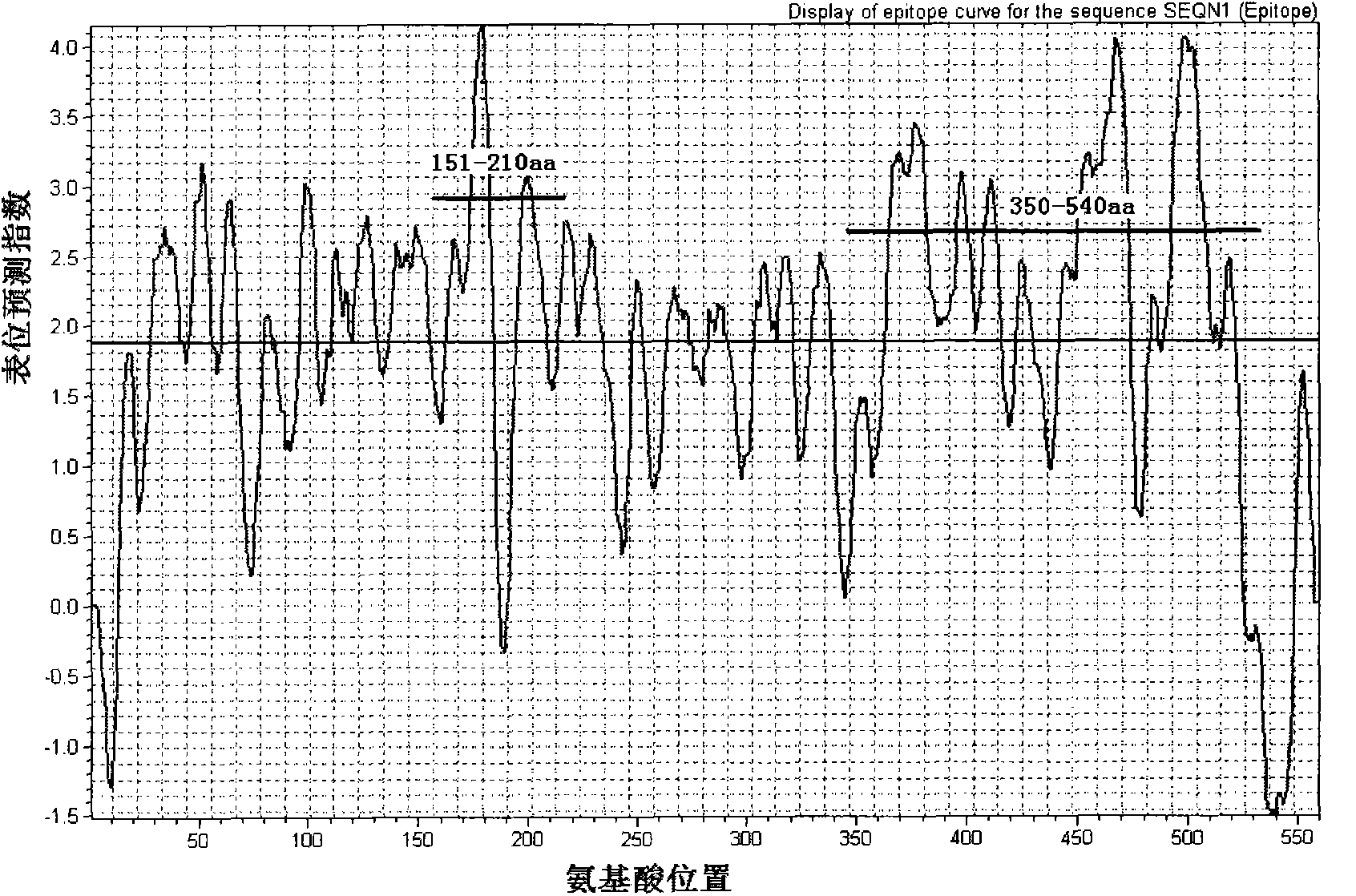
[0038] We used BioSun bioinformatics software to predict the antigenicity of human infected H7N9 avian influenza virus sequences. The results of HA and NA epitopes are as follows: figure 1 , figure 2 As shown, two main dominant antigen segments are selected for each antigen, and their amino acid sequences are respectively shown in sequence 1-sequence 4 in the sequence listing.
Embodiment 24
[0039] Cloning and expression of 24 kinds of human infection H7N9 avian influenza antigens
[0040] 1. Construction of four kinds of antigen expression plasmids of human infection with H7N9 avian influenza virus
[0041] 1. Synthesis of antigen genes of 14 human-infected H7N9 avian influenza viruses
[0042] According to the amino acid sequences of the above four human-infected H7N9 avian influenza antigens, Shanghai Yingjun Biotechnology Service Co., Ltd. was commissioned to synthesize the genes of the above sequences using the dominant codons of Escherichia coli. The gene sequences are shown in sequences 5-8 in the sequence table.
[0043] 1. Construction of expression plasmids for 24 human-infected H7N9 avian influenza antigens
[0044] 1.2.1 PCR product and expression vector pBVIL1 double digestion
[0045] Take 30 μl of the above synthetic gene product and pBVIL1 expression vector and put them in Eeppendorf centrifuge tubes respectively, add 4 μl of 10×buffer (H), 1 μl ...
Embodiment 3
[0053] Example 3 Establishment and Application of IgG Antibody Detection (Indirect Method) Technology Based on Recombinant Human Infection with H7N9 Avian Influenza Antigen
[0054] 3.1 Establishment of IgG antibody detection (indirect method) technology based on recombinant human infection with H7N9 avian influenza antigen
[0055] Dilute the purified recombinant human infected H7N9 with pH 7.2 phosphate buffer solution to 2.0 μg / ml, 100 μl per well, discard the solution after overnight at 4 °C, coat the ELISA assay plate, rinse with distilled water 3 times, pat dry, and remove each well. Add 1% BSA100μl, room temperature for 2h, discard the solution. After adding 100 μl sample dilute solution to each well, add 10 μl serum of the sample to be tested, 37°C for 30 minutes, discard the solution, wash the plate 5 times with washing solution, discard the solution, pat dry, add horseradish peroxidase-labeled anti-human IgG monoclonal antibody (1:1000) 100μl, incubate at 37°C for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com