Preparation of recombinant Huaxi crab metallothionein (rcmt) and its monoclonal antibody
A technology of metallothionein and Huaxi crab, which is applied in the biological field, can solve the problems of difficulty in establishing a fast, accurate and standardized enzyme-linked immunoassay method, poor specificity of polyclonal antibodies, and uneven physical and chemical properties, so as to improve stability And purity, strong specificity, highly uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
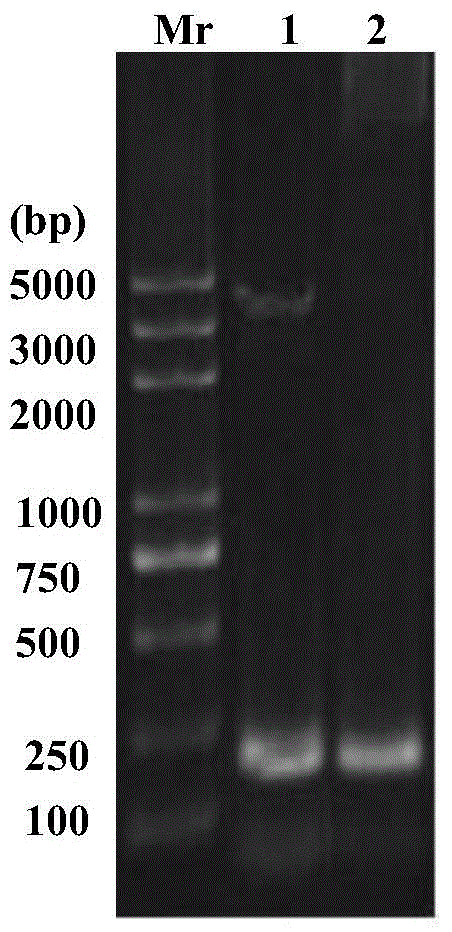
[0034] Example 1 Recombinant expression of metallothionein from Henan Huaxi crab.
[0035] Step 1: Selection of plasmid vector
[0036] The basic phosphate promoter (phoA) and signal sequence have several advantages in secreting and expressing foreign proteins in E. coli (see the third edition of "Molecular Cloning Experiment Guide"), which can maintain the natural conformation of foreign proteins to the greatest extent and at the same time secrete Proteins to the periplasm are more stable. In the phoA system, the phoA gene encodes the alkaline phosphatase of the phosphate scavenging system of Escherichia coli, which is inhibited in the presence of excess phosphate, and is gradually induced in the state of starvation (bacterial growth enters the lag phase). This regulation makes the accumulation of exogenous protein in the cell gradual, unlike the rapid accumulation regulated by other promoters. This chronic accumulation reduces the chances of overloading the bacterial expre...
Embodiment 2
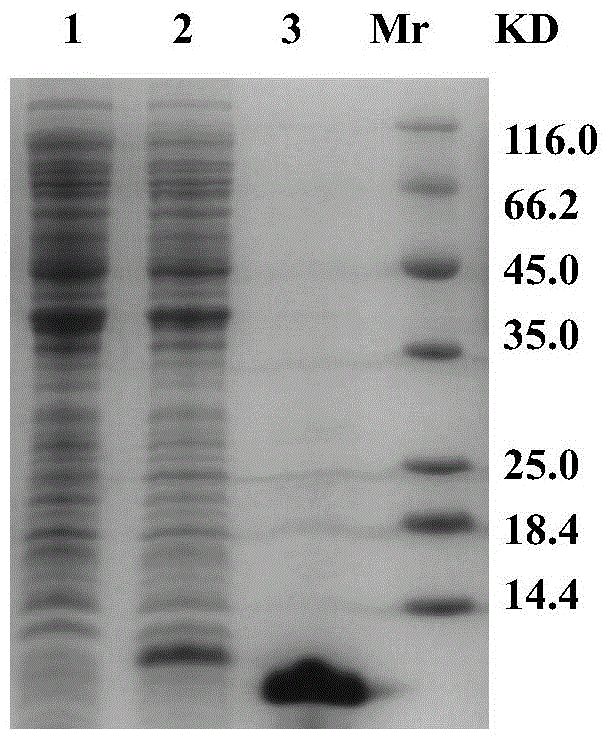
[0045] Example 2 Preparation of anti-Huaxi crab metallothionein monoclonal antibody
[0046] Step 1: Mouse Immunization
[0047] The purified rcMT uses BSA as a standard protein, and the protein concentration is determined by the BCA method.
[0048] (1) Basic immunity:
[0049] Dilute the rcMT stored in the buffer (0.01MTris-HCl, 0.2mMPMSF, pH7.8) to 120μg / ml, and mix it with Quicklyantibody water-soluble adjuvant (purchased from Beijing Kang Biquan Biotechnology Co., Ltd.) according to the volume ratio of 1:1. Mix well, and inject intramuscularly to immunize mice, the injection volume is 0.1ml / mouse. The amount of immunity is 0.1ml, 20μgrcMT / only. After the injection, the injection site is disinfected with alcohol cotton balls to prevent infection. (2) Booster immunization: 21 days later, the mice were immunized for the second time, and the amount and method of immunization were the same as above. (3) Shock immunity: 35d tail blood collection ELISA assay. When the serum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com