Preparation method for CZTS nano-particle material
A nanoparticle, copper-zinc-tin-sulfur technology, applied in the field of materials, can solve the problems of long reaction time and poor crystallinity, and achieve the effects of shortened reaction time, good crystallinity, and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
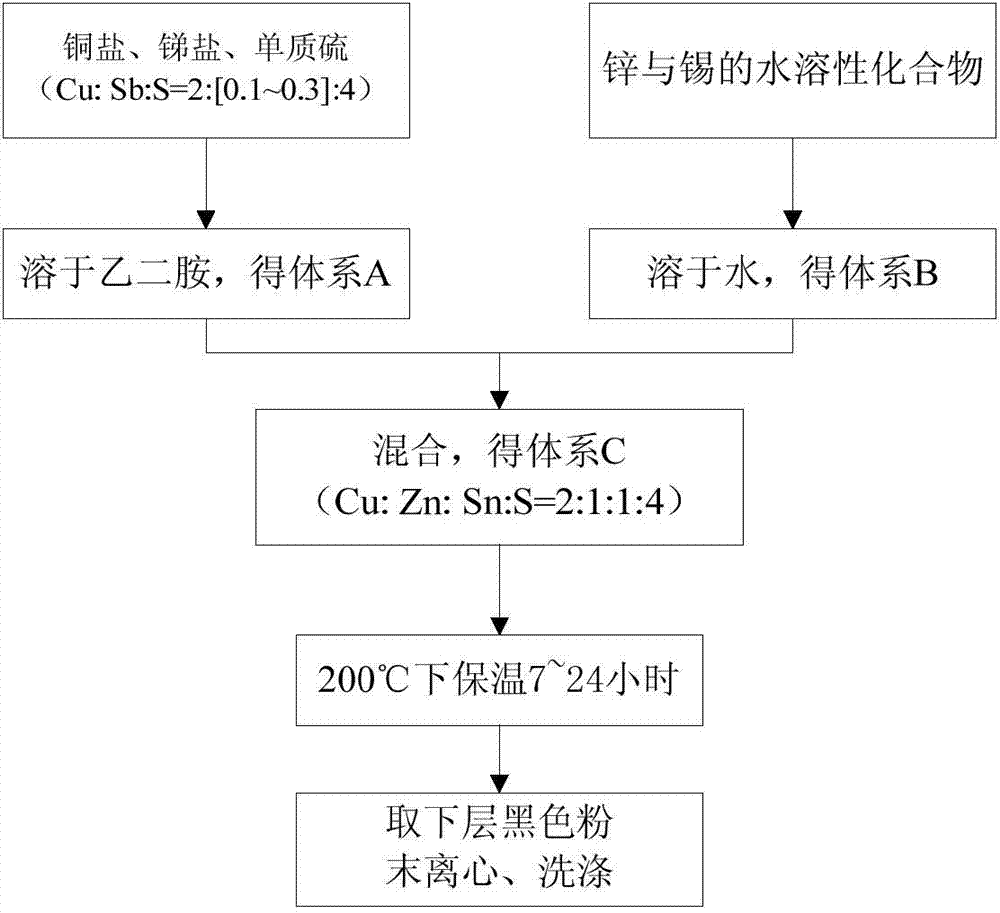
[0025] a. Prepare the following two solutions respectively:
[0026] Solution A: Weigh 0.02mol CuCl 2 , 0.04mol S, 0.001mol SbCl 3 In a 20ml polytetrafluoroethylene reactor filled with 10mol ethylenediamine, ultrasonically dissolve for 10 minutes.
[0027] Solution B: Weigh 0.01mol ZnCl 2 , 0.01molSnSO 4 In a 25ml beaker filled with 4ml deionized water, sonicate for 10 minutes.
[0028] b. Pour solution B into a 20ml reactor containing solution A, rinse the beaker with 2ml of water and pour it into the reactor, and ultrasonically dissolve the reactor containing 16ml of solution for 10 minutes.
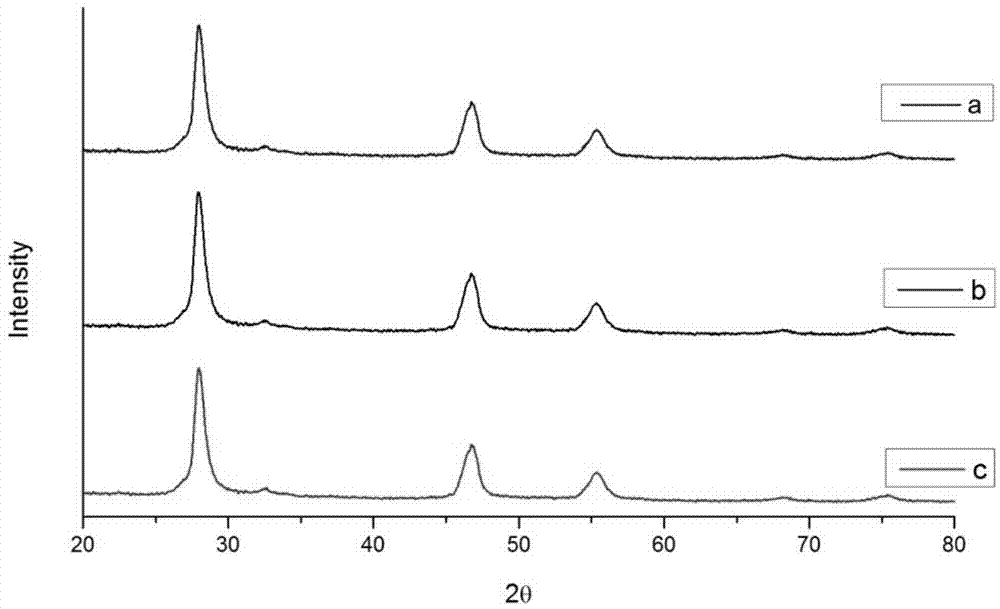
[0029] c. Seal the reaction kettle and put it into an oven, raise the temperature to 200°C, and keep it warm for 7 hours.
[0030] d. After the reaction kettle was cooled to room temperature, the black dispersion in the lower layer was taken out, and impurities were removed by centrifugal washing with ethanol three times to obtain copper-zinc-tin-sulfur nanoparticles.
Embodiment 2
[0032] a. Prepare the following two solutions respectively:
[0033] Solution A: Weigh 0.02mol Cu(NO 3 ) 2 , 0.04mol S, 0.002mol Sb(NO 3 ) 3 In a 20ml polytetrafluoroethylene reactor filled with 10mol ethylenediamine, ultrasonically dissolve for 10 minutes.
[0034] Solution B: Weigh 0.01mol ZnSO 4 , 0.01molSnSO 4 In a 25ml beaker filled with 4ml deionized water, sonicate for 10 minutes.
[0035] b. Pour solution B into a 20ml reaction kettle containing solution A, rinse the beaker with 2ml of water and pour it into the reaction kettle, and ultrasonically dissolve the reaction kettle with 16ml of solution for 10 minutes.
[0036] c. Seal the reaction kettle and put it into an oven, raise the temperature to 200°C, and keep it warm for 12 hours.
[0037] d. After the reaction kettle was cooled to room temperature, the lower black dispersion system was taken out, and impurities were removed by centrifugal washing with deionized water three times to obtain copper-zinc-tin-s...
Embodiment 3
[0039] a. Prepare the following two solutions respectively:
[0040] Solution A: Weigh 0.02mol Cu(acac) 2 (copper acetylacetonate), 0.04mol S, 0.002mol SbCl 3 In a 20ml polytetrafluoroethylene reactor filled with 10mol ethylenediamine, ultrasonically dissolve for 10 minutes.
[0041] Solution B: Weigh 0.01mol Zn(NO 3 ) 2 , 0.01molSnSO 4 In a 25ml beaker filled with 4ml deionized water, sonicate for 10 minutes.
[0042] b. Pour solution B into a 20ml reaction kettle containing solution A, rinse the beaker with 2ml of water and pour it into the reaction kettle, and ultrasonically dissolve the reaction kettle with 16ml of solution for 10 minutes.
[0043] c. Seal the reaction kettle and put it into an oven, raise the temperature to 200 degrees Celsius, and keep it warm for 24 hours.
[0044] d. After the reaction kettle was cooled to room temperature, the lower black dispersion system was taken out, and impurities were removed by centrifugal washing with deionized water thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com