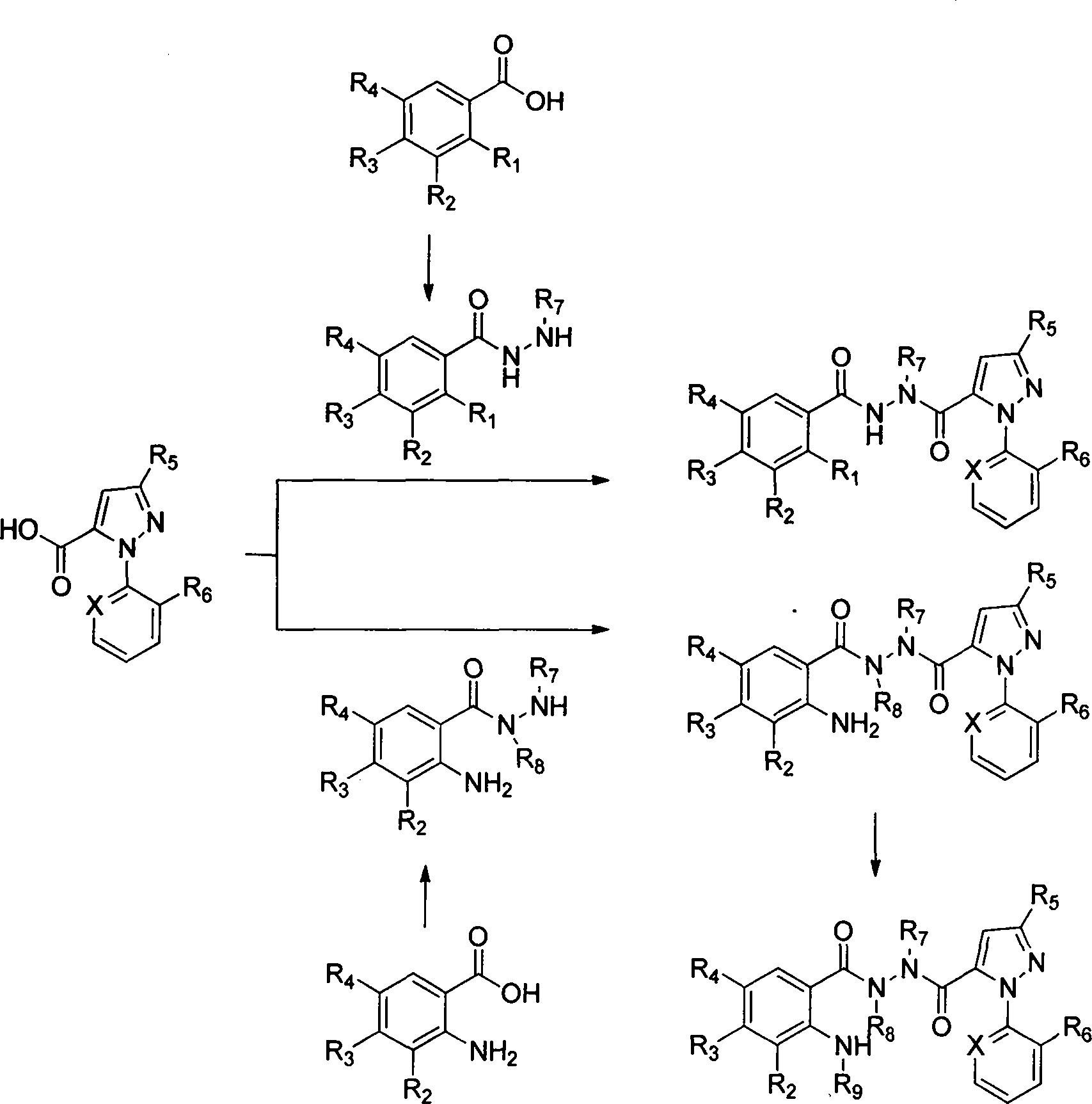
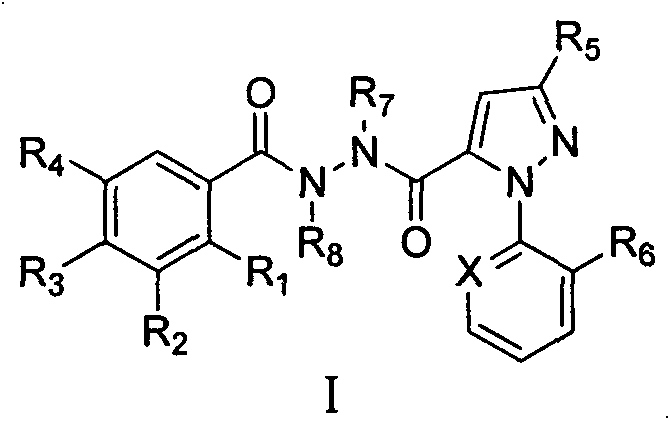
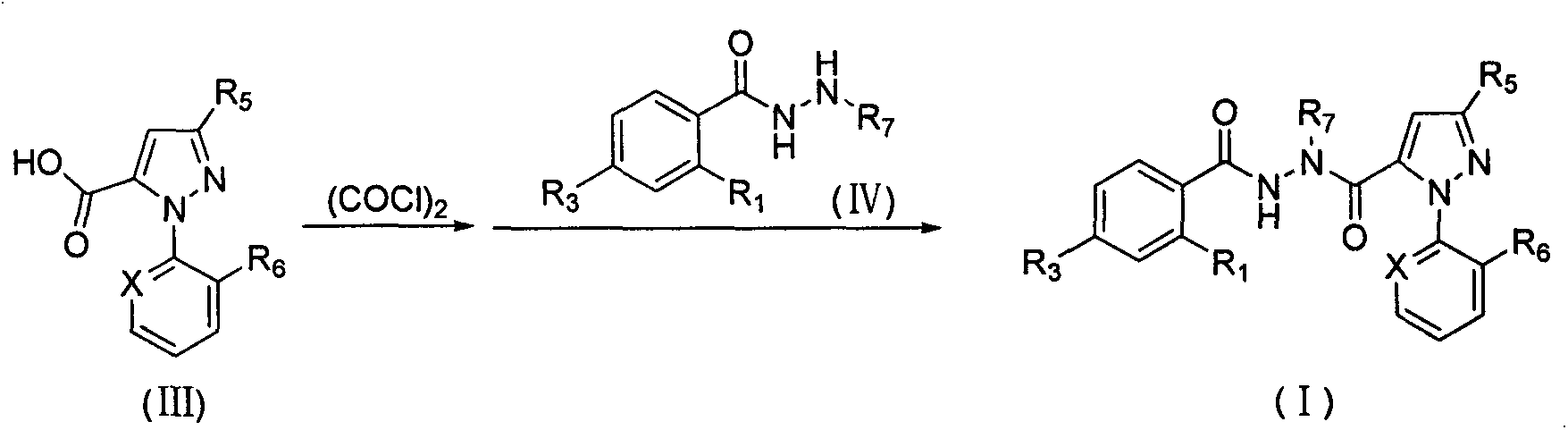
Novel hydrazide derivatives as well as preparation method and application thereof
A technology of hydrazide derivatives and derivatives, which is applied in the field of synthesis of agricultural chemical insecticides, can solve the problems of increased consumer costs and reduced productivity, and achieve excellent insecticidal activity, improved fat solubility, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 3-Bromo-N-tert-butyl-1-(3-chloro-2-pyridyl)-N'-(4-trifluoromethylbenzoyl)-1H-pyrazole-5-carboxhydrazide (derivatized Synthesis of Item 01):
[0045] Step A: Preparation of N’-tert-butyl-4-trifluoromethyl benzohydrazide
[0046] Add 4-trifluoromethylbenzoic acid (0.57g, 3mmol), SOCl in a 100mL round bottom flask 2 , Reflux, reduce excess SOCl 2 , The crude acid chloride is obtained, and it is added dropwise to a mixture of a suitable amount of tert-butylhydrazine hydrochloride, sodium hydroxide, dichloromethane and water. After dropping, react at room temperature, add ethyl acetate, separate the organic layer, and the organic layer After desolvation, the title compound was obtained, 0.57 g of white powder, mp 77-78°C.
[0047] Step B: Preparation of 3-bromo-N-tert-butyl-1-(3-chloro-2-pyridinyl)-N'-(4-trifluoromethylbenzoyl)-1H-pyrazole-5- Formyl hydrazide
[0048] Add 3-bromo-1-(3-chloro-2-pyridyl)-1H-biazole-5-carboxylic acid (0.30g, 1.00mm0l), oxalyl chloride and dichloromet...
Embodiment 2
[0050] N'-(2-Amino-5-chloro-3-methylbenzoyl)-3-bromo-N-tert-butyl-1-(3-chloro-2-pyridyl)-1H-pyrazole-5 -Synthesis of formyl hydrazide (derivative 17)
[0051] Step A: Preparation of 2-amino-3-methyl-5-chlorobenzoic acid
[0052] Dissolve 2-amino-3-methylbenzoic acid (15.10g, 100mmol) in DMF, add excess NCS to it, heat, cool, and pour into 500mL ice water. A brown-red solid appears. Filter and dry to obtain the title compound. 12.3g.
[0053] Step B: Preparation of N’-tert-butyl-2-amino-3-methyl-5-chlorobenzohydrazide
[0054] Add 2-amino-3-methyl-5-chlorobenzoic acid (0.56g, 3mmol), SOCl in a 100mL round bottom flask 2 , Reflux, reduce excess SOCl 2 , To obtain crude acid chloride, add dropwise to a mixture of tert-butylhydrazine hydrochloride, sodium hydroxide, dichloromethane and water under cooling in an ice-water bath, react at room temperature, add 100 mL of ethyl acetate, and separate the organic layer After the organic layer was desolvated, the title compound was obtained, 0....
Embodiment 3
[0058] N-[2-(2-(3-Bromo-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-formyl)-2-(tert-butyl)hydrazinocarbonyl)-4 -Chloro-6-methylphenyl] the synthesis of methyl carbamate (derivative 45)
[0059] Add N'-(2-amino-5-chloro-3-methylbenzoyl)-3-bromo-N-tert-butyl-1-(3-chloro-2-pyridine to a 100mL single-necked round bottom flask Base)-1H-pyrazole-5-carboxhydrazide (0.32g, 0.6mmol), triethylamine and dichloromethane, add acetyl chloride dropwise, after dropping, stir at room temperature, after the reaction is over, add ethyl acetate, divide The organic layer was separated, and after the organic layer was desolvated, the title compound was obtained, 0.14 g white powder, mp264-265°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com