NGR polypeptide radiopharmaceutical as well as preparation method and application thereof
A technology of radiopharmaceuticals and synthetic methods, which is applied in the field of NGR polypeptide radiopharmaceuticals and its preparation, can solve the problems of unclear mechanism and unexplained position of the label, etc., and achieve the effect of low liver uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
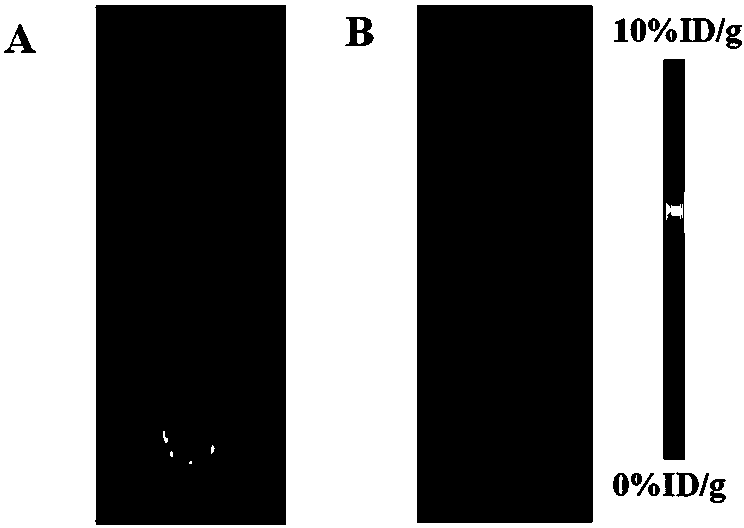
Image
Examples
Embodiment 1
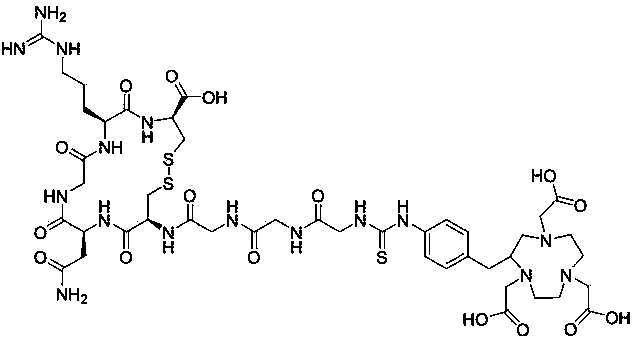
[0060] Example 1: 68 Preparation of Ga-NOTA-Gly3-NGR polypeptide radiopharmaceutical:
[0061] (1) Preparation of NOTA-Gly3-NGR:
[0062] Will p -SCN-Bn-NOTA (3.42 mg, 6.1 μmol) was dissolved in 25 μL dimethyl sulfoxide (DMSO), and Gly3-NGR polypeptide monomer (GGGCNGRC, 4-8 two cysteines connected to form a ring) was added ( 4.0 mg, 5.55 μmol), dissolve 20 μL of N,N-diisopropylethylamine (DIPEA) in 200 μL of N,N-dimethylformamide (DMF) solution, after reacting for 1 h, add 20 μL of acetic acid The reaction was stopped by 500 μL of the aqueous solution, and the reaction was stopped by Luna C18 (5 μm, 250 × 10 mm) semi-preparative column HPLC method (Waters HPLC, equipped with 2 515 pumps, 2487 UV detector, wavelength 214 nm and 254 nm, gradient elution 27.5 min, mobile phase A is 0.1% TFA aqueous solution, mobile phase B is 0.1% TFA acetonitrile solution, starting with 95% A, 5% B, and ending with 40% A, 60% B.) Separation Purify, collect the fractions with a retention time of a...
Embodiment 2
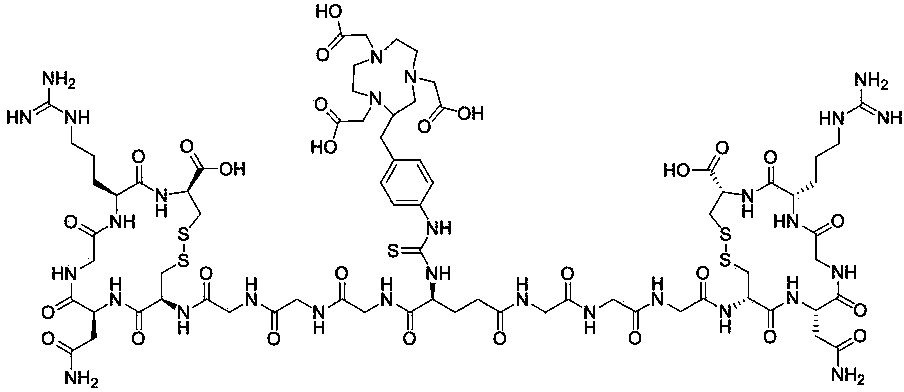
[0067] Example 2: 68 Preparation of Ga-NOTA-E(Gly3-NGR)2 polypeptide radiopharmaceutical:
[0068] (1) Synthesis of Boc-E(Gly3-NGR)2:
[0069] Boc-protected glutamate activated ester Boc-E(OSu)2 (2.05 mg, 4.6 μmol), Gly3-NGR peptide monomer (GGGCNGRC, 4-8 two cysteines linked to form a ring) (10.0 mg, 14 μmol) mixed and dissolved in DMF, adjusted the pH to 8-9, stirred at room temperature for 8-12 h, separated and purified by Luna C18 (5 μm, 250 × 10 mm) semi-preparative column HPLC method (same as Example 1), and collected retention time About 13 min fractions, the collected liquids were combined and lyophilized to obtain 5.1 mg; the obtained product was analyzed by ESI-MS mass spectrometry and the result was m / z C 58 H 93 N 25 O 24 S 4 [M+H] + =1652.65, the theoretical value is 1652.57. Confirmed as the expected product Boc-E(Gly3-NGR)2,
[0070] (2) Synthesis of E(Gly3-NGR)2:
[0071] Boc-E(Gly3-NGR)2 was dissolved in 0.5 ml trifluoroacetic acid:triisopropylsilane:water=95:2.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com