Terminal group-functionalized polyethylene and preparation method thereof
A technology of terminal double-bond polyethylene and polyethylene, which is applied in the field of terminal functionalized polyethylene and its preparation, can solve the problems of limited functionalization research, and achieve the effect of simple and efficient preparation method, clear structure and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
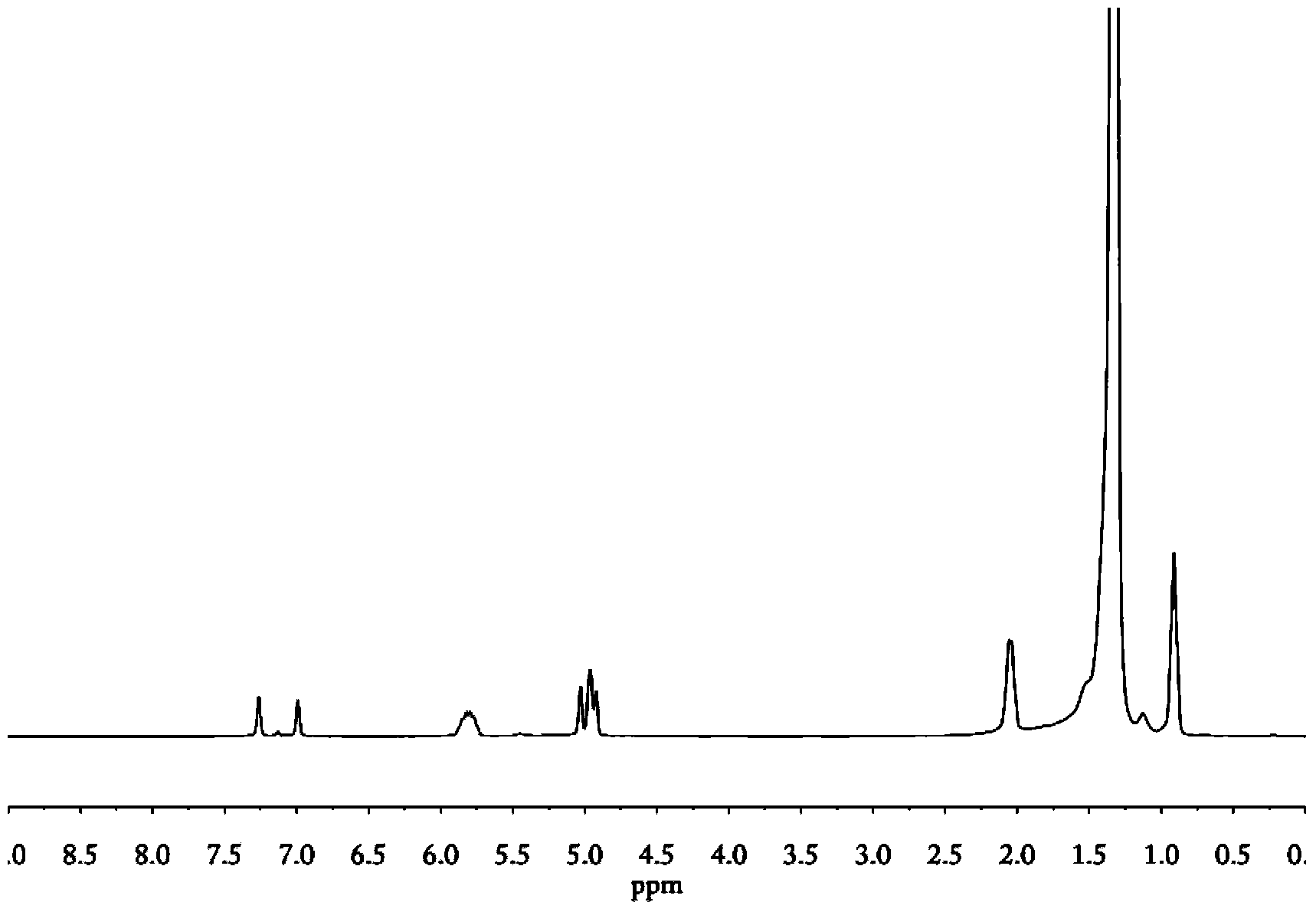
[0060] A 500ml dry three-neck flask was pumped three times successively with nitrogen and polymer grade ethylene gas, then filled with ethylene gas and injected with a certain amount of toluene, the iron-based catalyst (toluene solution) and methylaluminoxane (toluene solution), at 30 After reacting at ℃ for 30 minutes, the white solid was collected by filtration, washed with alcohol and deionized water, and dried under vacuum at 50℃ to obtain the initial terminal double-bond polyethylene (defined as "v-PE0").
[0061] The above v-PE0 was extracted with boiling n-hexane for 12 hours, and the solvent was removed to obtain terminal double bond polyethylene (defined as "v-PE1"); Double-bond polyethylene (defined as "v-PE2"; cyclohexane insoluble matter was collected to obtain terminal double-bond polyethylene (defined as "v-PE3".
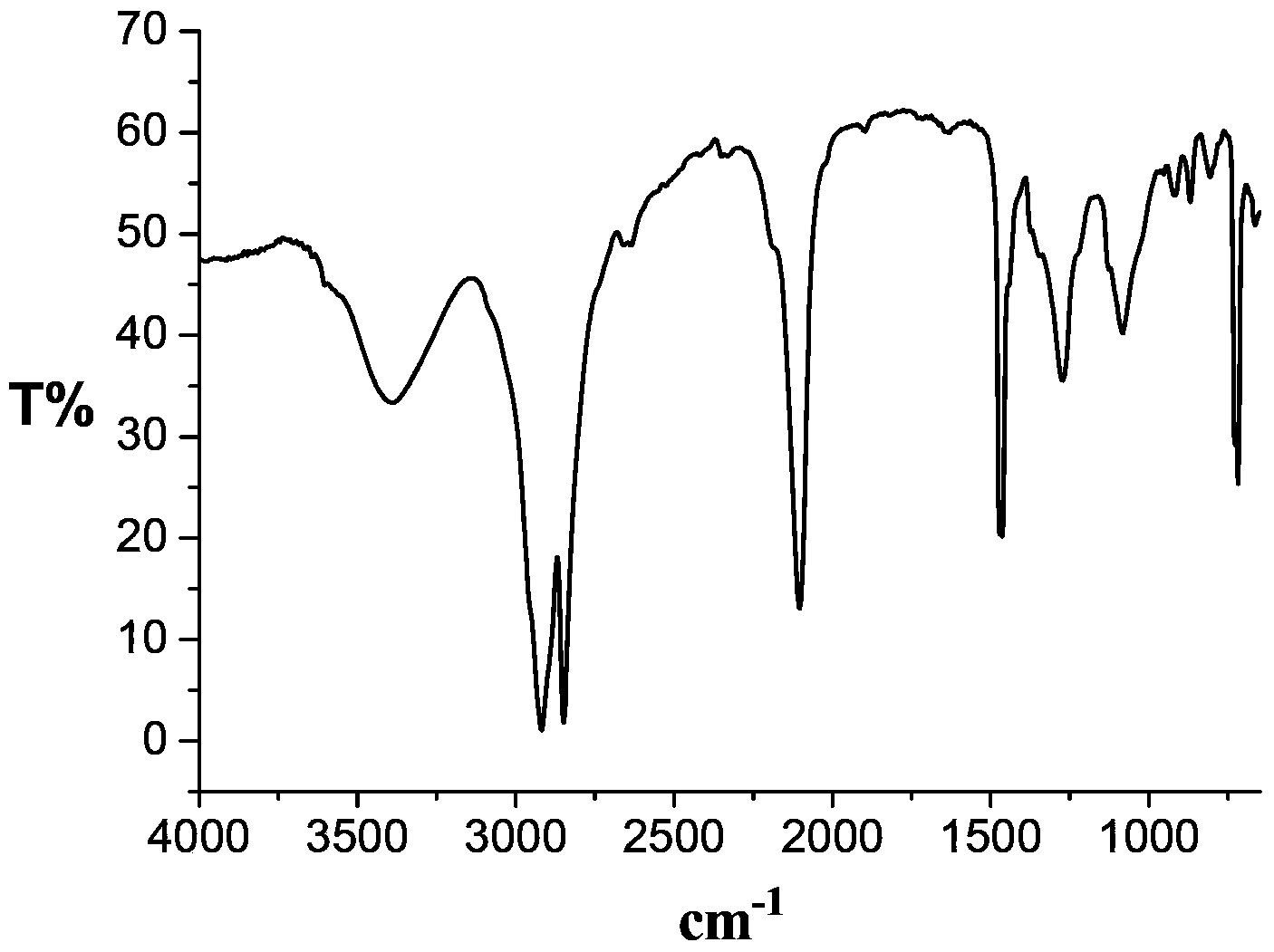
[0062] 1 H NMR and 13 C NMR characterization confirms that the above-mentioned terminal double-bond polyethylene v-PE (including v-PE0, v-PE1, v-PE2...
Embodiment 2
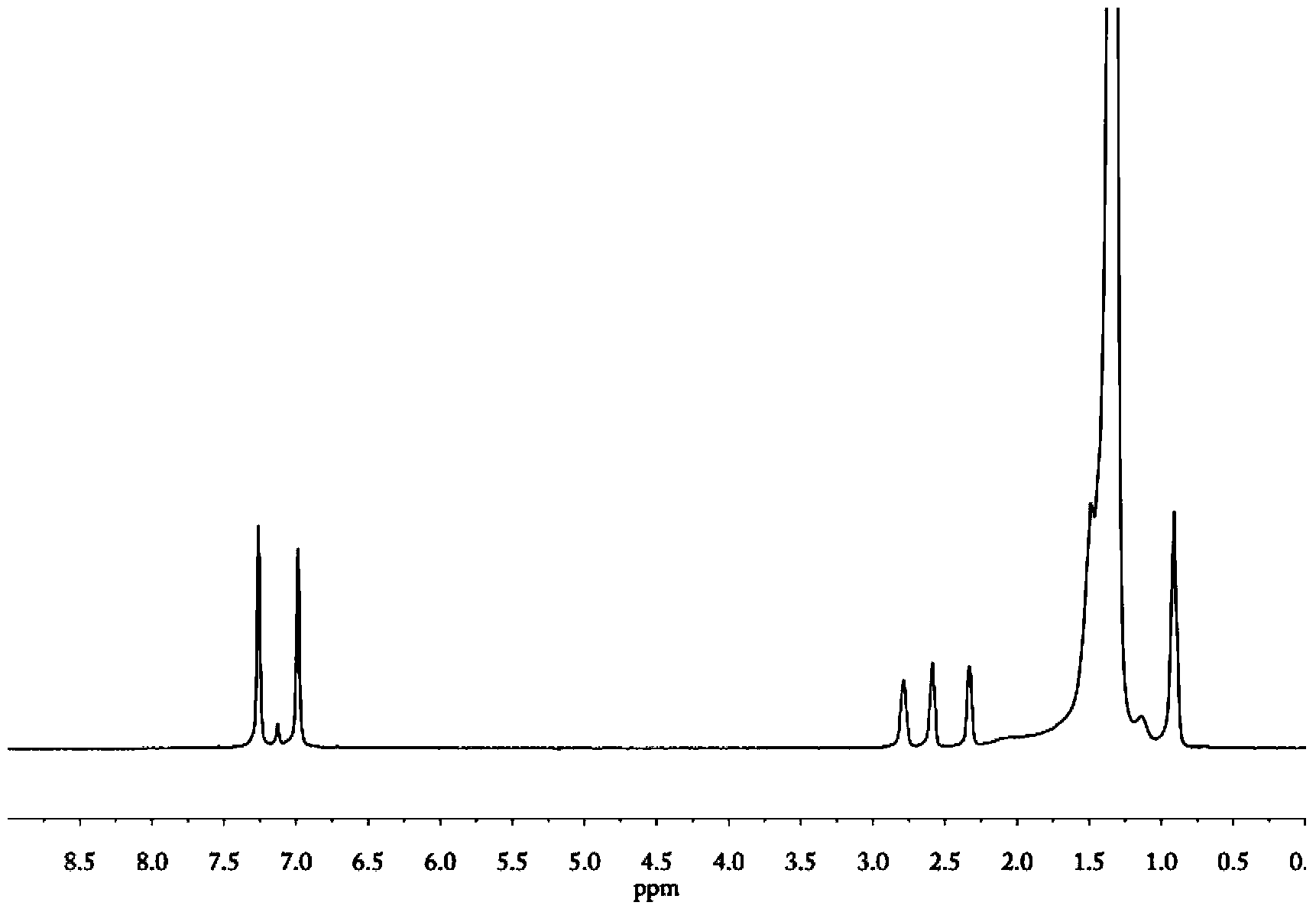
[0064] In a 250ml two-necked bottle, add 5g of v-PE0 described in Example 1, vacuum dry at 40°C for 1 hour, fill with nitrogen, add 70ml of xylene, heat up to 110°C, stir to fully dissolve. Add 0.5g phosphomolybdenum heteropolyacid X under nitrogen atmosphere 7 PMo 12 o 42 [X=N(CH 3 ) 4 ], 0.12g methyl trioctyl ammonium bisulfate. After stirring evenly, slowly add 5ml of 30wt% hydrogen peroxide dropwise. Continue to stir and react for 5 hours, precipitate the polymer with a large amount of methanol, filter, wash repeatedly with formic acid and dry in vacuum at 50°C for 12 hours to obtain epoxy group-terminated polyethylene, the structure of which is shown in formula (I) (R is structure a).
[0065] 1 H NMR and 13 C NMR characterization confirmed that the polyethylene terminal unsaturated double bonds were completely converted into epoxy groups.
Embodiment 3
[0067] In a 250ml two-necked bottle, add 4g of double-bond-terminated polyethylene v-PE1 described in Example 1, vacuum-dry at 40°C for 1 hour, fill with nitrogen, add 30ml of heptane, heat up to 65°C, and stir to fully dissolve it. Add 2g of m-chloroperbenzoic acid under a nitrogen atmosphere, react for 2 hours, precipitate the polymer with a large amount of methanol, filter, wash with formic acid repeatedly at 50°C and dry in vacuum for 12 hours to obtain epoxy group-terminated polyethylene, the structure of which is shown in formula (I) ( R is shown in structure a).
[0068] 1 H NMR and 13 C NMR characterization confirmed that the polyethylene terminal unsaturated double bonds were completely converted into epoxy groups.
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com